Assay preparation—
Place not fewer than 5 Boluses in a high-speed glass blender jar containing an accurately measured volume of water, and blend for 4 ± 1 minutes. Dilute an accurately measured volume of this stock solution quantitatively and stepwise with water to obtain an
Assay preparation containing about 1.25 mg of ampicillin per mL.
Procedure—
Proceed as directed for
Procedure under
Iodometric Assay—Antibiotics  425
425
. Calculate the quantity, in mg, of ampicillin (C
16H
19N
3O
4S) in each Bolus taken by the formula:
(
T /
D)(
F / 2000)(
B 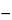 I
I),
in which
T is the labeled quantity, in mg, of ampicillin in each Bolus; and
D is the concentration, in mg per mL, of ampicillin in the
Assay preparation on the basis of the labeled quantity in each Bolus and the extent of dilution.