Assay preparation—
Transfer an accurately weighed quantity of Dental Paste, equivalent to about 1.5 mg of triamcinolone acetonide, to a screw-cap tube. Add 20.0 mL of
Internal standard solution, cap, and place in a sonic bath for 15 to 20 minutes. Place in a water bath at 70

for 5 minutes, then swirl for 1 minute. Repeat the heating and swirling sequence once. Cool in an ice–methanol bath for 15 minutes, then centrifuge for 10 minutes at
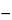
5

. Mix an accurately measured volume of the supernatant with an equal volume of
Mobile phase. Place in an ice–methanol bath for 15 minutes, then centrifuge for 15 minutes. Draw off and discard the upper phase. Filter the lower phase to obtain a clear solution.