Identification—
Place a 2-g quantity of Cream in a conical flask, add 50 mL of chloroform and 15 g of anhydrous sodium sulfate, and swirl to dissolve the specimen. Filter the solution and clarify the filtrate, if necessary, by the further addition of anhydrous sodium sulfate and a second filtration. Evaporate the filtrate to near dryness, and dissolve the residue in chloroform to obtain a solution containing about 100 µg per mL. Apply 10 µL of this solution and 10 µL of a solution of
USP Triamcinolone Acetonide RS in chloroform containing 100 µg per mL, on a line parallel to and about 1.5 cm from the bottom edge of a thin-layer chromatographic plate (see
Chromatography  621
621
) coated with a 0.25-mm layer of chromatographic silica gel. Place the plate in a developing chamber containing and equilibrated with a mixture of chloroform, benzene, and methanol (100:40:20). Develop the chromatogram until the solvent front has moved about 12 cm above the line of application. Remove the plate, allow the solvent to evaporate, and spray with a mixture of equal volumes of sodium hydroxide solution (1 in 5) and a 1 in 500 solution of blue tetrazolium in methanol: the intensity of the blue color and the
RF of the spot obtained with the solution under test are similar to those of the spot obtained with the Standard solution.
Assay—
Mobile phase—
Prepare a solution of acetonitrile in water containing approximately 30% (v/v) of acetonitrile.
Internal standard solution—
Dissolve fluoxymesterone in isopropyl alcohol to obtain a solution having a concentration of about 50 µg per mL.
Assay preparation—
Transfer an accurately weighed quantity of Cream, equivalent to about 1.5 mg of triamcinolone acetonide, to a screw-cap tube. Add 20.0 mL of
Internal standard solution, and cap securely. Heat for 5 minutes at 60

, then swirl vigorously for not less than 30 seconds. Repeat the heating and swirling sequence three times. Cool in a methanol-ice bath for 15 to 20 minutes, then centrifuge for 15 minutes at
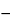
5

. Dilute an accurately measured volume of the supernatant with an equal volume of
Mobile phase. Cool in a methanol-ice bath for 10 to 15 minutes, with occasional agitation. Filter first through a pledget of glass wool or a prefilter disk and then through a 0.45-µm porosity membrane to obtain a clear solution.
Procedure—
Introduce equal volumes (between 15 and 25 µL) of the
Assay preparation and the
Standard preparation into a high-pressure liquid chromatograph (see
Chromatography  621
621
), operated at room temperature, by means of a suitable microsyringe or sampling valve. Adjust the operating parameters with
Mobile phase on the column, such that the separation of triamcinolone acetonide and internal standard is optimized, with a retention time of about 14.5 minutes for triamcinolone acetonide. Typically, the apparatus is fitted with a 30-cm × 4-mm column containing packing L1, and is equipped with a UV detector capable of monitoring absorbance at 254 nm, and a suitable recorder. In a suitable chromatogram, the coefficient of variation for five replicate injections of a single specimen is not more than 3.0%, and the resolution factor,
R (see
Chromatography  621
621
), between the peaks for triamcinolone acetonide and fluoxymesterone is not less than 2.0. Measure the heights of the internal standard and triamcinolone acetonide peaks, at the same retention times obtained from the
Assay preparation and the
Standard preparation. Calculate the quantity, in mg, of C
24H
31FO
6 in the portion of Cream taken by the formula:
40C(RU / RS),
in which
C is the concentration, in mg per mL, of
USP Triamcinolone Acetonide RS in the
Standard preparation, and
RU and
RS are the ratios of the peak heights of triamcinolone acetonide to the internal standard obtained from the
Assay preparation and the
Standard preparation, respectively.