Identification—
Chill 10 filled containers to about
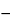
75

in a dry ice-acetone mixture for 15 to 20 minutes. Carefully remove the top of each container with a tube cutter, allow to stand for 15 minutes, and pour the contents into a 100-mL beaker. Pour about 5 mL of the combined contents into a 100-mL beaker containing 50 mL of chloroform, shake, and filter through a medium-porosity sintered-glass funnel. Wash the residue with five 10-mL portions of chloroform. Allow the residue to dry by drawing air through the funnel: the IR absorption spectrum of a potassium bromide dispersion of the residue so obtained exhibits maxima only at the same wavelengths as that of a similar preparation of
USP Terbutaline Sulfate RS.
Water, Method I  921
921 —
—
Transfer the contents of a weighed container to the titration vessel by attaching the valve stem to an inlet tube. Weigh the empty container, and determine the weight of the specimen taken. The
Water content, determined by
Method I  921
921
, is not more than 0.02%.
Delivered dose uniformity over the entire contents:
meets the requirements for
Metered-Dose Inhalers under
Aerosols, Nasal Sprays, Metered-Dose Inhalers, and Dry Powder Inhalers  601
601
.
PROCEDURE FOR DOSE UNIFORMITY—
4-Aminoantipyrine solution—
On the day of use, prepare a solution of 4-aminoantipyrine in water having a concentration of 20 mg per mL.
Potassium ferricyanide solution—
On the day of use, prepare a solution of potassium ferricyanide in water having a concentration of 80 mg per mL.
Standard preparation—
Dissolve an accurately weighed quantity of
USP Terbutaline Sulfate RS in water, and dilute quantitatively and stepwise with water to obtain a solution having a known concentration of about 20 µg of terbutaline sulfate per mL.
Test preparation—
Discharge the minimum recommended dose into the sampling apparatus and detach the inhaler as directed. Rinse the apparatus (filter and interior) with four 4.0-mL portions of 0.01 N sulfuric acid, and quantitatively transfer the resulting solutions to a 50-mL centrifuge tube. Wash the apparatus (filter and interior) with 10 mL of chloroform, and add the washing to the solution in the centrifuge tube. Wash the apparatus (filter and interior) with 4.0 mL of 0.01 N sulfuric acid, and quantitatively transfer the resulting liquid to the same centrifuge tube. Shake vigorously for 1 minute, and centrifuge for 10 minutes. Use the clear aqueous phase as the Test preparation.
Procedure—
Pipet 2 mL of the
Test preparation, 2 mL of the
Standard preparation, and 2 mL of water to serve as a reagent blank, into separate 1-cm stoppered cells. To each cell add 0.5 mL of
4-Aminoantipyrine solution, and mix. Add 0.5 mL of
Potassium ferricyanide solution to each cell, and mix. Thirty seconds, accurately timed, after the addition of the
Potassium ferricyanide solution, determine the absorbances of the solutions against the blank, at the wavelength of maximum absorbance at about 550 nm. Calculate the quantity, in µg, of (C
12H
19NO
3)
2·H
2SO
4 contained in the minimum dose taken by the formula:
10CN(AU / AS),
in which
C is the concentration, in µg per mL, of
USP Terbutaline Sulfate RS in the
Standard preparation; N is the number of sprays discharged to obtain the minimum dose; and
AU and
AS are the absorbances of the solutions from the
Test preparation and the
Standard preparation, respectively, corrected for the absorbances of the reagent blank solution.
Assay—
Mobile phase—
Prepare a solution containing 750 mL of water, 140 mL of methanol, 110 mL of tetrahydrofuran, and 1.08 g of sodium 1-octanesulfonate. Filter and degas. Make adjustments if necessary (see
System Suitability under
Chromatography  621
621
).
System suitability solution—
Dissolve suitable quantities of
USP Terbutaline Sulfate RS and 3,5-dihydroxy-
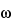
-
tert-butylaminoacetophenone sulfate in water to obtain a solution containing about 50 and 20 µg per mL, respectively.
Standard preparation—
Dissolve an accurately weighed quantity of
USP Terbutaline Sulfate RS in water to obtain a solution having a known concentration of about 0.3 mg per mL.
Assay preparation—
Accurately weigh not less than three containers, and separately perform the following procedure for each of the units. Chill in a dry ice-acetone mixture to about
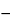
75

for 15 to 20 minutes. Quickly and carefully remove the top of the container with a tube cutter. Allow the propellants to evaporate at room temperature for 10 to 15 minutes.
[NOTE—Avoid complete evaporation of the propellants.
] Quantitatively transfer the suspension to a 500-mL separatory funnel with the aid of chloroform. Wash all parts of the container alternately with several small portions of chloroform followed by small portions of 0.01 N sulfuric acid. Transfer the washings to the separatory funnel, and adjust the phase volumes to about 100 mL each with chloroform and 0.01 N sulfuric acid, respectively. Dry the container and all of its parts at 105

for 1 hour. Cool to room temperature, and weigh. Shake the separatory funnel for 1 minute, allow the phases to separate, and discard the chloroform layer. Filter the acidic aqueous phase through filter paper into a 250-mL volumetric flask. Wash the separatory funnel with two 10-mL portions of water, and transfer the washings to the volumetric flask. Dilute with water to volume, mix, and filter, discarding the first 2 mL of the filtrate.
Chromatographic system
(see
Chromatography  621
621
)—The liquid chromatograph is equipped with a 280-nm detector and a 6.2-mm × 8-cm column that contains 3-µm packing L7 and is maintained at 40

, and fitted with a 0.5-µm pre-column. The flow rate is about 1.5 mL per minute. Chromatograph the
System suitability solution, and record the peak responses as directed for
Procedure: the relative retention times are about 0.83 and 1.0 for terbutaline and 3,5-dihydroxy-
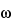
-
tert-butylaminoacetophenone, respectively; and the resolution,
R, between terbutaline sulfate and 3,5-dihydroxy-
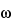
-
tert-butylaminoacetophenone is not less than 1.6. Chromatograph the
Standard preparation, and record the peak responses as directed in the
Procedure: the relative standard deviation for replicate injections is not more than 2.0%.
Procedure—
Separately inject equal volumes (about 20 µL) of the
Standard preparation and the
Assay preparation into the chromatograph, record the chromatograms, and measure the responses for the major peaks. Calculate the quantity, in mg, of (C
12H
19NO
3)
2·H
2SO
4 in each container taken by the formula:
250C(rU / rS),
in which
C is the concentration, in mg per mL, of
USP Terbutaline Sulfate RS in the
Standard preparation, and
rU and
rS are the peak responses obtained from the
Assay preparation and
Standard preparation, respectively.