Packaging and storage—
Preserve in well-closed, light-resistant containers.
Identification—
A:
A 1 in 2000 solution in dilute sulfuric acid (1 in 350) exhibits a vivid blue fluorescence. On the addition of a few drops of hydrochloric acid, the fluorescence disappears.
B:
In the test for
Chromatographic purity, the
RF value of the principal spot obtained from the
Test preparation corresponds to that from the
Standard preparation.
C:
A solution (1 in 50) made with the aid of a few drops of hydrochloric acid responds to the tests for
Sulfate  191
191
.
Specific rotation  781S
781S :
:
between
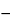
235

and
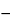
245

.
Test solution:
20 mg per mL, in 0.1 N hydrochloric acid.
Heavy metals, Method II  231
231 :
:
0.001%.
Chloroform-alcohol-insoluble substances—
Warm 2 g with 15 mL of a mixture of chloroform and dehydrated alcohol (2:1) at about 50

for 10 minutes. Filter through a tared, sintered-glass filter, using gentle suction. Wash the filter with five 10-mL portions of the chloroform-alcohol mixture, dry at 105

for 1 hour, and weigh: the weight of the residue does not exceed 2 mg (0.1%).
Chromatographic purity—
Diluted standard preparation—
Dilute a portion of the Standard preparation with diluted alcohol to a concentration of 0.06 mg per mL.
Related substances preparation—
Prepare a solution in diluted alcohol containing in each mL 0.05 mg each of
USP Quininone RS (corresponding to 0.06 mg of the sulfate), and 0.10 mg of cinchonidine (corresponding to 0.12 mg of the sulfate).
Test preparation—
Prepare a solution of Quinine Sulfate in diluted alcohol to contain 6 mg per mL.
Procedure—
Apply 10-µL portions of the
Test preparation, the
Standard preparation, the
Diluted standard preparation, and the
Related substances preparation to a suitable thin-layer chromatographic plate (see
Chromatography  621
621
) coated with a 0.25-mm layer of chromatographic silica gel. Allow to dry, and develop the chromatogram in a solvent system consisting of a mixture of chloroform, acetone, and diethylamine (5:4:1), the solvent chamber being used without previous equilibration. When the solvent front has moved about 15 cm, remove the plate from the chamber, mark the solvent front, and allow the solvent to evaporate. Spray the chromatogram with glacial acetic acid. Locate the spots on the plate by examination under long-wavelength UV light. Any spot produced by the
Test preparation at the
RF value of a spot produced by the
Related substances preparation is not greater in size or intensity than that corresponding spot. Apart from these spots and from the spot appearing at the
RF value of Quinine Sulfate, any additional fluorescent spot is not greater in size or intensity than the spot of the
Diluted standard preparation. Spray the plate with
potassium iodoplatinate TS. Any spot produced by the
Test preparation is not greater in size or intensity than a corresponding spot from the
Related substances preparation.
Limit of dihydroquinine sulfate—
Methanesulfonic acid solution—
Add 35.0 mL of methanesulfonic acid to 20.0 mL of glacial acetic acid, dilute with water to 500 mL, and mix.
Diethylamine solution—
Dissolve 10.0 mL of diethylamine in water to obtain 100 mL of solution.
Mobile phase—
Prepare a suitable filtered and degassed mixture of water, acetonitrile, Methanesulfonic acid solution, and Diethylamine solution (860:100:20:20). Adjust with Diethylamine solution to a pH of 2.6 if found to be lower.
System suitability preparation—
Transfer about 10 mg each of quinine sulfate and dihydroquinine to a 50-mL volumetric flask. Dissolve in about 5 mL of methanol, dilute with Mobile phase to volume, and mix.
System suitability test—
Chromatograph injections of the System suitability preparation as directed for Procedure: the relative retention times for quinine and dihydroquinine are about 1 and 1.5, respectively. The resolution between the quinine and dihydroquinine peaks is not less than 1.2. The relative standard deviation for the peak response of quinine is not more than 2.0%.
Test preparation—
Transfer about 20 mg of Quinine Sulfate to a 100-mL volumetric flask, dissolve in and dilute with Mobile phase to volume, and mix.
Procedure
(see
Chromatography  621
621
)—Inject about 50 µL of the
Test preparation into a chromatograph equipped with a 235-nm detector and a 3.9-mm × 30-cm column that contains packing L1. The response of the dihydroquinine peak is not greater than one-ninth that of the quinine peak (10.0%).
Organic volatile impurities, Method IV  467
467 :
:
meets the requirements.
Assay—
Dissolve about 200 mg of Quinine Sulfate, accurately weighed, in 20 mL of acetic anhydride, add 4 drops of p-naphtholbenzein TS, and titrate with 0.1 N perchloric acid VS from a 10-mL microburet to a green endpoint. Perform a blank determination, and make any necessary correction. Each mL of 0.1 N perchloric acid is equivalent to 24.90 mg of total alkaloid salt, calculated as (C20H24N2O2)2·H2SO4.