Packaging and storage—
Preserve in tight, light-resistant containers.
Labeling—
Label it to indicate whether it is obtained from natural sources or is prepared synthetically.
Angular rotation  781A
781A :
:
between
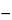
0.15

and +0.15

.
Aldehydes and ketones—
Shake 10 mL with 50 mL of a saturated solution of sodium bisulfite in a graduated cylinder, and allow the mixture to stand for 6 hours: no appreciable diminution in the volume of Anethole occurs, and no crystalline deposit separates.
Limit of phenols—
Shake 1 mL with 20 mL of water, and allow the liquids to separate. Pass the water layer through a filter paper previously moistened with water, and to 10 mL of the filtrate add 3 drops of
ferric chloride TS: no purple or purplish color is produced.