Calibration—
The constants
A and
B are determined by operating the instrument with the U-tube filled with two different samples of known density (e.g., degassed water and air). Perform the control measurements daily, using degassed water: the results displayed for the control measurement using degassed water do not deviate from the reference value (
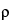 25
25 = 0.997043 g/cm
–3) by more than its specified error. Precision is a function of the repeatability and stability of the oscillator frequency. Density meters are able to achieve measurements with an error on the order of 1×10
–3g/cm
–3 to 1×10
–5g/cm
–3 and a repeatability of 1×10
–4g/cm
–3 to 1×10
–6g/cm
–3. For example, an instrument specified to ±1×10
–4g/cm
–3 must display 0.9970 ± 0.0001 g/cm
–3 in order to be suitable for further measurement, otherwise a readjustment is necessary. Calibration with certified reference materials should be carried out regularly.
Procedure—
Using the manufacturer's instructions, perform the measurements using the same procedure as for
Calibration. If necessary, equilibrate the liquid to be examined at 25

before introduction into the tube to avoid the formation of bubbles and to reduce the time required for measurement. Factors affecting accuracy include the following:
-
temperature uniformity throughout the tube,
-
nonlinearity over a range of density,
-
parasitic resonant effects, and
-
viscosity, if the oscillating transducer density meters used do not provide automatic compensation of sample viscosity influence.
 USP29
USP29