Procedure—
Proceed as directed for
Procedure under
Iodometric Assay—Antibiotics  425
425
. Calculate the quantity, in mg, of ampicillin (C
16H
19N
3O
4S) in each Tablet taken by the formula:
(
T/
D)(
F/2000)(
B 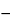 I
I),
in which
T is the labeled quantity, in mg, of ampicillin in each Tablet; and
D is the concentration, in mg per mL, of ampicillin in the
Assay preparation on the basis of the labeled quantity in each Tablet and the extent of dilution.