Chromatographic system (see Chromatography  621
621 )—
)—
The liquid chromatograph is equipped with a refractive index detector and three 7.5-mm × 30-cm columns containing packing L38, and maintained at a constant temperature. Chromatograph the
Marker solution, and record the peak responses as directed for
Procedure: the elution profile shows two peaks, the first due to the
Vo marker, the second due to dextrose. Determine the void volume,
Vo, of the system as the inflection point of the ascending part of the first peak. Determine the total volume,
VT , of the system as the maximum of the second peak; the tailing factor,
t, of the dextrose peak is not more than 1.3; and the relative standard deviation of the ratio
Vo / VT is not more than 1%. Chromatograph each of the
Calibration solutions separately, and record the peak responses as directed for
Procedure. Divide each profile into at least 60 vertical sections of equal volume increments. (The actual number of sections is represented by the variable
a in the equations below.) Record
yi, the height above the baseline, corresponding to each value of
vi, the volume eluted at that section. For each value of
vi, calculate the distribution coefficient,
Ki, by the formula:
(
vi 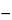 Vo
Vo) / (
VT 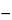 Vo
Vo).
Find appropriate values of
b1,
b2,
b3,
b4, and
b5, using a suitable method,
* that, when substituted in the equation:
and the resulting values of
Mi substituted, along with their corresponding values of
yi, in the equation:
give values of weight average molecular weight,
bar(M)w , within 5% of the labeled values for each of the
Calibration solutions and 180 ± 2 for dextrose. Chromatograph the
System suitability solution, and record the peak responses as directed for
Procedure. Calculate
bar(M)w of the total molecular weight distribution using the same method as directed for the
Calibration solutions, but inserting the now known values of
b1,
b2,
b3,
b4, and
b5. It is between 39,000 and 46,000.
Similarly, calculate bar(M)w of the high-fraction dextran eluted through section n by the formula:
in which n is defined by the relations:
It is between 111,000 and 135,000.
Similarly, calculate bar(M)w of the low-fraction dextran eluted in and after section m by the formula:
in which m is defined by:
It is between 6000 and 9000.
Procedure—
Chromatograph a 50-µL volume of the
Test solution, and record the peak responses. Calculate values of the weight average molecular weight,
bar(M)w , of the total molecular weight distribution of the high-fraction dextran, and of the low-fraction dextran as directed for
System Suitability under
Chromatography  621
621
the values are between 35,000 and 45,000, not more than 120,000, and not less than 5,000, respectively. With the values of
b1,
b2,
b3,
b4, and
b5, obtained with the
Calibration solutions under
Chromatographic system, calculate the number average molecular weight,
bar(M)n, of the total molecular weight distribution of the
Test solution by substituting the corresponding values of
Mi, along with their corresponding values of
yi, in the equation:
The number average molecular weight,
bar(M)n, is between 16,000 and 30,000. Where Dextran 40 is labeled as intended for use in the preparation of injectables, the ratio
bar(M)w /
bar(M)n is in the 1.4 to 1.9 range.