Packaging and storage—
Preserve in well-closed containers.
Labeling—
Label Solution to state the solvent used and the claimed concentration of anhydrous aluminum chlorohydrate contained therein.
Identification—
A:
A solution containing the equivalent of about 100 mg of anhydrous aluminum chlorohydrate per mL responds to the tests for
Aluminum  191
191
and for
Chloride  191
191
.
B:
Identification of propylene glycol (where stated on the label)—Add about 10 mL of isopropyl alcohol to 2 g of Solution, mix, and filter. Evaporate the filtrate to about 1 mL on a steam bath: the IR spectrum of a film of this solution on a silver chloride disk exhibits maxima only at the same wavelengths as that of a similar preparation of a film of propylene glycol.
C:
Identification of dipropylene glycol (where stated on the label)—Add about 10 mL of isopropyl alcohol to 2 g of Solution, mix, and filter. Evaporate the filtrate to about 1 mL on a steam bath: the IR spectrum of a film of this solution on a silver chloride disk exhibits maxima only at the same wavelengths as that of a similar preparation of a film of dipropylene glycol.
D:
Identification of alcohol (where stated on the label)—In a small beaker mix 5 drops of Solution with 1 mL of potassium permanganate solution (1 in 100) and 5 drops of 2 N sulfuric acid. Immediately cover the beaker with filter paper moistened with a freshly prepared solution of 0.1 g of sodium nitroferricyanide and 0.25 g of piperazine in 5 mL of water: an intense blue color is produced on the filter paper, the color fading after a few minutes.
pH  791
791 :
:
between 3.0 and 5.0, in a solution prepared by diluting 3 g of the solution with water to obtain 10 mL.
Arsenic, Method I  211
211 —
—
Prepare the
Test Preparation using an accurately weighed quantity of the Solution. The limit is 2 ppm.
Heavy metals, Method I  231
231 —
—
Prepare the
Test Preparation using an accurately weighed quantity of the Solution. The limit is 0.001%.
Limit of iron—
Standard preparation—
Transfer 2.0 mL of
Standard Iron Solution, prepared as directed under
Iron  241
241
, to a 50-mL beaker.
Test preparation—
Transfer 5.3 g of the Solution to a 100-mL volumetric flask, dilute with water to volume, and mix. Transfer 5.0 mL of this solution to a 50-mL beaker.
Procedure—
To each of the beakers containing the
Standard preparation and the
Test preparation add 5 mL of 6 N nitric acid, cover with a watch glass, and boil on a hot plate for 3 to 5 minutes. Allow to cool, add 5 mL of
Ammonium Thiocyanate Solution, prepared as directed under
Iron  241
241
, transfer to separate 50-mL color comparison tubes, dilute with water to volume, and mix: the color of the solution from the
Test preparation is not darker than that of the solution from the
Standard preparation (75 µg per g).
Content of aluminum—
Edetate disodium titrant—
Prepare and standardize as directed in the
Assay under
Ammonium Alum, except to use 37.2 g of edetate disodium, instead of 18.6 g.
Test solution—
Transfer about 400 mg of the Solution, accurately weighed, to a 250-mL beaker, add 20 mL of water and 5 mL of hydrochloric acid, and boil on a hot plate for not less than 5 minutes, and allow to cool.
Procedure—
To the
Test solution add 25.0 mL of
Edetate disodium titrant, and adjust with 2.5 N ammonium hydroxide or 1 N acetic acid to a pH of 4.7 ± 0.1. Add 20 mL of acetic acid-ammonium acetate buffer TS, 50 mL of alcohol, and 5 mL of
dithizone TS. The pH of this solution should be 4.7 ± 0.1. Titrate with 0.1
M zinc sulfate VS until the color changes from green-violet to rose-pink. Perform a blank titration, and make any necessary correction. Each mL of 0.1 M
Edetate disodium titrant consumed is equivalent to 2.698 mg of aluminum (Al). Calculate the percentage of aluminum (Al) found, and use this value to calculate the
Aluminum/
chloride atomic ratio.
Content of chloride—
Transfer about 1.4 g of the Solution, accurately weighed, to a 250-mL beaker, and add 100 mL of water and 10 mL of diluted nitric acid with stirring. Titrate with 0.1 N silver nitrate VS using a silver-silver chloride glass electrode and a silver billet electrode system, determining the endpoint potentiometrically. Each mL of 0.1 N silver nitrate is equivalent to 3.545 mg of chloride (Cl). Calculate the percentage of chloride (Cl) found, and use this value to calculate the
Aluminum/
chloride atomic ratio.
Aluminum/chloride atomic ratio—
Divide the percentage of aluminum found in the test for
Content of aluminum by the percentage of chloride found in the test for
Content of chloride and multiply by 35.453/26.98, in which 35.453 and 26.98 are the atomic weights of chlorine and aluminum, respectively: the ratio is between 1.91:1 and 2.10:1.
Assay—
Calculate the percentage of anhydrous aluminum chlorohydrate in the Solution by the formula:
Al{[26.98
x + (17.01)(3
x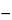
1) + 35.453] / 26.98
x},
in which
Al is the percentage of aluminum found in the test for
Content of aluminum,
x is the aluminum/chloride atomic ratio, 26.98 is the atomic weight of aluminum, 17.01 is the molecular weight of the hydroxide ion (OH), and 35.453 is the atomic weight of chloride (Cl).