Packaging and storage—
Preserve in tight containers.
Identification—
A:
Place about 3 g of finely powdered Tablets in a flask equipped with a stopper and glass tubing, the tip of which is immersed in
calcium hydroxide TS in a test tube. Add 5 mL of 3 N hydrochloric acid to the flask, and immediately insert the stopper: gas evolves in the flask and a precipitate is formed in the test tube.
B:
To the solution in the flask obtained in
Identification test
A add 5 drops of
methyl red TS, and heat to boiling. Add 6 N ammonium hydroxide until the color of the solution changes to deep yellow, continue boiling for 2 minutes, and filter through hardened filter paper. (Retain the filtrate for
Identification test
C.) Wash the precipitate with 350 mL of a hot ammonium chloride solution (1 in 50), discarding the washings: the precipitate so obtained, dissolved in 3 N hydrochloric acid, responds to the tests for
Aluminum  191
191
.
C:
The filtrate obtained in
Identification test
B responds to the tests for
Magnesium  191
191
.
Disintegration  701
701 :
:
10 minutes, simulated gastric fluid TS being substituted for water in the test.
Assay for aluminum hydroxide—
Edetate disodium titrant—
Prepare and standardize as directed in the
Assay under
Ammonium Alum.
Assay preparation—
Weigh and finely powder not fewer than 20 Tablets. Transfer an accurately weighed portion of the powder, equivalent to about 1200 mg of aluminum hydroxide, to a 150-mL beaker, add 20 mL of water, stir, and slowly add 30 mL 3 N hydrochloric acid. Heat gently, if necessary, to aid solution, cool, and filter into a 200-mL volumetric flask. Wash the filter with water into the flask, add water to volume, and mix.
Procedure—
Pipet 10 mL of the
Assay preparation into a 250-mL beaker, add 20 mL of water, then add, in the order named and with continuous stirring, 25.0 mL of
Edetate disodium titrant and 20 mL of acetic acid–ammonium acetate buffer TS, and heat near the boiling point for 5 minutes. Cool, add 50 mL of alcohol and 2 mL of
dithizone TS, and mix. Titrate with 0.05 M zinc sulfate VS until the color changes from green-violet to rose-pink. Perform a blank determination, substituting 10 mL of water for the
Assay preparation, and make any necessary correction. Each mL of 0.05
M Edetate disodium titrant consumed is equivalent to 3.900 mg of Al(OH)
3.
Assay for magnesium carbonate—
Weigh and finely powder not fewer than 20 Tablets. Transfer an accurately weighed portion of the powder, equivalent to about 750 mg of magnesium carbonate, to a 250-mL conical flask fitted with a two-hole stopper. Fill the lower transverse section of a U-shaped drying tube of about 15-mm internal diameter and 15-cm height with loosely packed glass wool. Place in one arm of the tube about 5 g of anhydrous calcium chloride, and accurately weigh the tube and the contents. Into the other arm of the tube place 9.5 g to 10.5 g of soda lime, and again weigh accurately. Insert stoppers in the open arms of the U-tube, and connect the side tube of the arm filled with soda lime to a calcium chloride drying tube, which in turn is connected to one of the holes in the stopper of the 250-mL conical flask. Attach a dropping funnel to the other hole in the stopper of the 250-mL conical flask. Add 100 mL of water and 10 mL of a mixture of hydrochloric acid and nitric acid (4:1) to the 250-mL conical flask through the dropping funnel, and close the dropping funnel. Heat the 250-mL conical flask at 95

for 1 hour, and allow the evolved carbon dioxide to pass through the U-tube. Replace the dropping funnel with a source of carbon dioxide-free air, and pass the carbon dioxide-free air through the apparatus at a rate of about 75 mL per minute for 30 minutes. Disconnect the U-tube, cool to room temperature, remove the stoppers, and weigh. The increase in weight corresponds to the quantity of carbon dioxide evolved. Calculate the quantity, in mg, of magnesium carbonate in each Tablet taken by the formula:
(84.31 / 44.01)(I)(WA / WP),
in which 84.31 and 44.01 are the molecular weights of magnesium carbonate and carbon dioxide, respectively;
I is the quantity, in mg, of carbon dioxide evolved from the portion of Tablets taken;
WA is the average weight, in g, of 1 Tablet; and
WP is the weight, in g, of the portion of Tablets taken.
Assay for magnesium oxide—
Weigh and finely powder not fewer than 20 Tablets. Transfer an accurately weighed portion of the powder, equivalent to about 1000 mg of magnesium carbonate and magnesium oxide combined, to a beaker, add 20 mL of water, and slowly add 40 mL of 3 N hydrochloric acid, with mixing. Heat the mixture to boiling, cool, and filter into a 200-mL volumetric flask. Wash the beaker with water, adding the washings to the filter. Add water to volume, and mix. Transfer 20.0 mL of this solution to a 400-mL beaker, add 180 mL of water and 20 mL of triethanolamine, and stir. Add 10 mL of ammonia–ammonium chloride buffer TS and 3 drops of an eriochrome black indicator solution prepared by dissolving 200 mg of eriochrome black T in a mixture of 15 mL of triethanolamine and 5 mL of dehydrated alcohol, and mix. Cool the solution to between 3

and 4

by immersion of the beaker in an ice bath, then remove, and titrate with 0.05
M edetate disodium VS to a blue endpoint. Perform a blank determination, substituting 20 mL of water for the assay solution, and make any necessary correction. Each mL of 0.05
M edetate disodium consumed is equivalent to 1.216 mg of Mg. Calculate the quantity, in mg, of magnesium equivalent in each Tablet taken by the formula:
10T(WA / WP),
in which
T is the magnesium equivalent obtained in the titration;
WA is the average weight, in g, of 1 Tablet; and
WP is the weight, in g, of the portion of Tablets taken. Calculate the quantity, in mg, of magnesium oxide in each Tablet taken by the formula:
(40.30 / 24.31)(
A 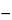
0.2883
B),
in which 40.30 and 24.31 are the molecular weight of magnesium oxide and the atomic weight of magnesium, respectively;
A is the quantity, in mg, of magnesium equivalent in each Tablet; and
B is the quantity, in mg, of magnesium carbonate in each Tablet, as determined in the
Assay for magnesium carbonate.