 726
726 ELECTROPHORESIS
ELECTROPHORESIS
Electrophoresis refers to the migration of electrically charged proteins, colloids, molecules, or other particles when dissolved or suspended in an electrolyte through which an electric current is passed.
Based upon the type of apparatus used, electrophoretic methods may be divided into two categories, one called free solution or moving boundary electrophoresis and the other called zone electrophoresis.
In the free solution method, a buffered solution of proteins in a U-shaped cell is subjected to an electric current which causes the proteins to form a series of layers in order of decreasing mobility, which are separated by boundaries. Only a part of the fastest moving protein is physically separated from the other proteins, but examination of the moving boundaries using a schlieren optical system provides data for calculation of mobilities and information on the qualitative and quantitative composition of the protein mixture.
In zone electrophoresis, the sample is introduced as a narrow zone or spot in a column, slab, or film of buffer. Migration of the components as narrow zones permits their complete separation. Remixing of the separated zones by thermal convection is prevented by stabilizing the electrolyte in a porous matrix such as a powdered solid, or a fibrous material such as paper, or a gel such as starch, agar, or polyacrylamide.
Various methods of zone electrophoresis are widely employed. Gel electrophoresis, particularly the variant called disk electrophoresis, is especially useful for protein separation because of its high resolving power.
Gel electrophoresis, which is employed by the compendium, is discussed in more detail following the presentation of some theoretical principles and methodological practices, which are shared in varying degrees by all electrophoretic methods.
The electrophoretic migration observed for particles of a particular substance depends on characteristics of the particle, primarily its electrical charge, its size or molecular weight, and its shape, as well as characteristics and operating parameters of the system. These latter include the pH, ionic strength, viscosity and temperature of the electrolyte, density or cross-linking of any stabilizing matrix such as gel, and the voltage gradient employed.
Effect of Charge, Particle Size, Electrolyte Viscosity, and Voltage Gradient—
Electrically charged particles migrate toward the electrode of opposite charge, and molecules with both positive and negative charges move in a direction dependent on the net charge. The rate of migration is directly related to the magnitude of the net charge on the particle and is inversely related to the size of the particle, which in turn is directly related to its molecular weight.
Very large spherical particles, for which Stokes' law is valid, exhibit an electrophoretic mobility, u0, which is inversely related to the first power of the radius as depicted in the equation:
where
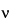
is the velocity of the particle,
E is the voltage gradient imposed on the electrolyte,
Q is the charge on the particle,
r is the particle radius, and
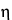
is the viscosity of the electrolyte. This idealized expression is strictly valid only at infinite dilution and in the absence of a stabilizing matrix such as paper or a gel.
Ions, and peptides up to molecular weights of at least 5000, particularly in the presence of stabilizing media, do not obey Stokes' law, and their electrophoretic behavior is best described by an equation of the type:
where A is a shape factor generally in the range of 4 to 6, which shows an inverse dependence of the mobility on the square of the radius. In terms of molecular weight, this implies an inverse dependence of mobility on the 2/3 power of the molecular weight.
Effect of pH—
The direction and rate of migration of molecules containing a variety of ionizable functional groups, such as amino acids and proteins, depends upon the pH of the electrolyte. For instance, the mobility of a simple amino acid such as glycine varies with pH approximately as shown in
Figure 1.
The pK
a values of 2.2 and 9.9 coincide with the inflection points of the sigmoid portions of the plot. Since the respective functional groups are 50% ionized at the pH values where pH = pK
a, the electrophoretic mobilities at these points are half of the value observed for the fully ionized cation and anion obtained at very low and very high pH, respectively. The zwitterion that exists at the intermediate pH range is electrically neutral and has zero mobility.
Effect of Ionic Strength and Temperature—
Electrophoretic mobility decreases with increasing ionic strength of the supporting electrolyte. Ionic strength, µ, is defined as:
where
Ci is the concentration of an ion in moles per L and
Zi is its valence, and the sum is calculated for all ions in the solution. For buffers in which both the anion and cation are univalent, ionic strength is identical with molarity.
Ionic strengths of electrolytes employed in electrophoresis commonly range from about 0.01 to 0.10. A suitable strength is somewhat dependent on the sample composition, since the buffer capacity must be great enough to maintain a constant pH over the area of the component zones. Zones become sharper or more compact as ionic strength is increased.
Temperature affects mobility indirectly, since the viscosity,
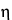
, of the supporting electrolyte is temperature-dependent. The viscosity of water decreases at a rate of about 3% per

C in the range of 0

to 5

and at a slightly lower rate in the vicinity of room temperature. Mobility, therefore, increases with increasing electrolyte temperature.
Considerable heat is evolved as a result of current passing through the supporting electrolyte. This heat increases with the applied voltage and with increasing ionic strength. Particularly in larger apparatus, despite the circulation of a coolant, this heat produces a temperature gradient across the bed which may lead to distortion of the separated zones. Therefore, practical considerations and the design of the particular apparatus dictate the choice of ionic strength and operating voltage.
Effect of a Stabilizing Medium, Electroosmosis—
When an electrical current is passed through an electrolyte contained in a glass tube or contained between plates of glass or plastic, a bulk flow of the electrolyte toward one of the electrodes is observed. This flow is called electroosmosis. It results from the surface charge on the walls of the apparatus, which arises either from ionizable functional groups inherent in the structural material or from ions adsorbed on the cell walls from the electrolyte contacting them. The effect is usually increased when the cell is filled with a bed of porous substance, such as a gel, used to stabilize the supporting electrolyte and prevent remixing of separated zones by thermal convection or diffusion. The solution immediately adjacent to the surface builds up an electrical charge, equal but opposite to the surface charge, and the electrical field traversing the cell produces a movement of solution toward the electrode of opposite charge.
The substances commonly used as stabilizing media in zone electrophoresis develop a negative surface charge, and therefore electroosmotic flow of the electrolyte is toward the cathode. As a result, all zones, including neutral substances, are carried toward the cathode during the electrophoretic run.
The degree of electroosmosis observed varies with the stabilizing substance. It is appreciable with agar gel, while it is negligibly small with polyacrylamide gel.
Molecular Sieving—
In the absence of a stabilizing medium or in cases where the medium is very porous, electrophoretic separation of molecules results from differences in the ratio of their electrical charge to their size. In the presence of a stabilizing medium, differences in adsorptive or other affinity of molecules for the medium introduces a chromatographic effect that may enhance the separation.
If the stabilizing medium is a highly cross-linked gel such that the size of the resultant pores is of the order of the dimensions of the molecules being separated, a molecular sieving effect is obtained. This effect is analogous to that obtained in separations based on gel permeation or molecular exclusion chromatography, but in gel electrophoresis the effect is superimposed on the electrophoretic separation. Molecular sieving may be visualized to result from a steric barrier to the passage of larger molecules. Small molecules pass through pores of a wide size range, and therefore their electrophoretic passage through the gel will not be impeded. As size increases, fewer pores will permit passage of the molecules, causing a retardation of the migration of substances of large molecular weight.
Gel Electrophoresis
Processes employing a gel such as agar, starch, or polyacrylamide as a stabilizing medium are broadly termed gel electrophoresis. The method is particularly advantageous for protein separations. The separation obtained depends upon the electrical charge to size ratio coupled with a molecular sieving effect dependent primarily on the molecular weight.
Polyacrylamide gel has several advantages that account for its extensive use. It has minimal adsorptive properties and produces a negligible electroosmotic effect. Gels of a wide range of pore size can be reproducibly prepared by varying the total gel concentration (based on monomer plus cross-linking agent) and the percentage of cross-linking agent used to form the gel. These quantities are conveniently expressed as
where T is the total gel concentration in %; C is the percentage of cross-linking agent used to prepare the gel; V is the volume, in mL, of buffer used in preparing the gel; and a and b are the weights, in g, of monomer (acrylamide) and cross-linking agent (usually N,N¢-methylenebisacrylamide) used to prepare the gel. Satisfactory gels ranging in concentration (T) from about 3% to 30% have been prepared. The amount of cross-linking agent is usually about one-tenth to one-twentieth of the quantity of monomer (C = 10% to 5%), a smaller percentage being used for higher values of T.
In the preparation of the gel, the bed of the electrophoresis apparatus is filled with an aqueous solution of monomer and cross-linking agent, usually buffered to the pH desired in the later run, and polymerized in place by a free radical process. Polymerization may be initiated by a chemical process, frequently using ammonium persulfate plus N,N,N¢,N¢-tetramethylenediamine or photochemically using a mixture of riboflavin and N,N,N¢,N¢-tetramethylenediamine. Polymerization is inhibited by molecular oxygen and by acidic conditions. The gel composition and polymerization conditions chosen must be adhered to rigorously to ensure reproducible qualities of the gel.
Apparatus for Gel Electrophoresis—
In general, the bed or medium in which electrophoresis is carried out may be supported horizontally or vertically, depending upon the design of the apparatus. A series of separations to be compared may also be carried out in several individual tubes or by placing different samples in adjacent wells, cast or cut into a single slab of gel. A vertical slab assembly such as that depicted schematically in
Figure 2
Fig. 2. Vertical Slab Gel Electrophoresis Apparatus.
is convenient for direct comparison of several samples. A particular advantage derives from the comparison of the samples in a single bed of gel which is likely to be more uniform in composition than gels cast in a series of chambers.
A feature of many types of apparatus, not illustrated in the schematic view, seals the lower buffer chamber to the base of the bed and allows the level of the buffer in the lower chamber to be made equal to that in the upper chamber, thereby eliminating hydrostatic pressure on the gel. In addition, some units provide for the circulation of coolant on one or both sides of the gel bed.
In the preparation of the gel, the base of the gel chamber is closed with a suitable device and the unit is filled with the solution of monomer, cross-linking agent, and catalyst. A comb, having teeth of an appropriate size, is inserted in the top, and polymerization is allowed to proceed to completion. Removal of the comb leaves a series of sample wells in the polymerized gel.
In simple gel electrophoresis, an identical buffer is used to fill the upper and lower buffer chambers as well as in the solution used to prepare the gel. After filling the chambers, the samples, dissolved in sucrose or other dense and somewhat viscous solution to prevent diffusion, are introduced with a syringe or micropipet into the bottoms of the sample wells, and the electrophoresis is begun immediately thereafter.
DISK ELECTROPHORESIS
An important variant of polyacrylamide gel electrophoresis, which employs a discontinuous series of buffers and often also a discontinuous series of gel layers, is called disk electrophoresis. The name is derived from the discoid shape of the very narrow zones that result from the technique. As a result of the narrow zones produced, this technique exhibits an extremely high resolving power and is to be recommended for the characterization of protein mixtures and for the detection of contaminants that may have mobilities close to that of the major component.
The basis of disk electrophoresis is outlined in the following paragraphs with reference to an anionic system suitable for separating proteins bearing a net negative charge. To understand disk electrophoresis, it is essential to have a knowledge of the general aspects of electrophoresis and the apparatus already described.
Basis of Disk Electrophoresis—
The high resolution obtained in disk electrophoresis depends on the use of a buffer system that is discontinuous with respect to both pH and composition. This is usually combined with a discontinuous series of two or three gels that differ in density.
A typical system is illustrated schematically in
Figure 3.
Fig. 3. Terminology, Buffer pH, and Buffer Composition for Acrylamide Gel Disk Electrophoresis.
A high density (T = 10% to 30%) separating gel several centimeters high is polymerized in a tris-chloride buffer in the bed of the apparatus. During polymerization the buffer is overlayered with a thin layer of water to prevent fixation of a meniscus in the top of the gel. The overlayer of water is then removed and a thin layer, 3 mm to 10 mm thick, of low density (T = 3%) gel, called the spacer or stacking gel, is polymerized in a tris-chloride buffer on top of the separating gel. An overlayer of water is again used to ensure a flat surface. The sample is mixed with a small amount of the spacer gel monomer solution which is applied on top of the spacer gel and allowed to polymerize. The pH of the separating gel is typically 8.9, while that of the spacer and sample gels is 6.7. All three gels are prepared using chloride as the anion.
The upper and lower buffer reservoirs are filled with a pH 8.3 buffer prepared from tris and glycine. At this pH about 3% of the glycine molecules bear a net negative charge.
When a voltage is applied across the system, the glycinate-chloride interface moves downward toward the anode. It was initially positioned at the junction of the buffer in the upper reservoir and the top of the sample gel layer. The chloride anion, by virtue of its small size, migrates faster than any of the proteins present in the sample. The pH of the sample and spacer layers was chosen to be about 3 units below the higher pKa of glycine. Therefore, in traversing these layers, only about 0.1% of the glycine molecules bear a net negative charge. Consequently, glycine migrates more slowly than chloride. The tendency for the faster-moving chloride to move away from glycinate lowers the concentration at the interface, producing a greater voltage drop at the interface, which in turn causes the glycinate to catch up to the chloride. Under these conditions, a very sharp interface is maintained, and as it moves through the sample and spacer layers, the proteins in the sample tend to stack themselves at the interface in very thin layers in order of mobility. The process is called stacking and is the source of the disks which are separated.
When the stacked proteins reach the high-density separating gel, they are slowed down by a molecular sieving process. The higher pH encountered in the running gel also causes the glycinate to migrate faster, so that the discontinuous buffer interface overtakes the proteins and eventually reaches the bottom of the separating gel. During this period, the disks of protein continue to separate by electrophoresis and molecular sieving in the separating gel. At the end of the run, the pH of the separating gel will have risen above its original value of 8.9 to a value of about pH 9.5.
Relative Mobility—
Bromophenol blue is often used as a standard for calculating the relative mobility of separated zones and to judge visually the progress of a run. It may be added to one of the sample wells, or mixed with the sample itself, or simply added to the buffer in the upper sample reservoir.
Relative mobility, MB, is calculated as:
Visualization of Zones—
Since polyacrylamide is transparent, protein bands may be located by scanning in a densitometer with UV light. The zones may be fixed by immersing in protein precipitants such as phosphotungstic acid or 10% trichloroacetic acid. A variety of staining reagents including naphthalene black (amido black) and Coomassie brilliant blue R250 may be used. The fixed or stained zones may be conveniently viewed and photographed with transmitted light from an X-ray film illuminator.
SAFETY PRECAUTIONS
Voltages used in electrophoresis can readily deliver a lethal shock. The hazard is increased by the use of aqueous buffer solutions and the possibility of working in damp environments.
The equipment, with the possible exception of the power supply, should be enclosed in either a grounded metal case or a case made of insulating material. The case should have an interlock that deenergizes the power supply when the case is opened, after which reactivation should be prevented until activation of a reset switch is carried out.
High-voltage cables from the power supply to the apparatus should preferably be a type in which a braided metal shield completely encloses the insulated central conductor, and the shield should be grounded. The base of the apparatus should be grounded metal or contain a grounded metal rim which is constructed in such a way that any leakage of electrolyte will produce a short which will deenergize the power supply before the electrolyte can flow beyond the protective enclosure.
If the power supply contains capacitors as part of a filter circuit, it should also contain a bleeder resistor to ensure discharge of the capacitors before the protective case is opened. A shorting bar that is activated by opening the case may be considered as an added precaution.
Because of the potential hazard associated with electrophoresis, laboratory personnel should be completely familiar with electrophoresis equipment before using it.