Where limits are expressed numerically herein, the upper and lower limits of a range include the two values themselves and all intermediate values, but no values outside the limits. The limits expressed in monograph definitions and tests, regardless of whether the values are expressed as percentages or as absolute numbers, are considered significant to the last digit shown.
Equivalence Statements in Titrimetric Procedures—
The directions for titrimetric procedures conclude with a statement of the weight of the analyte that is equivalent to each mL of the standardized titrant. In such an equivalence statement, it is to be understood that the number of significant figures in the concentration of the titrant corresponds to the number of significant figures in the weight of the analyte. Blank corrections are to be made for all titrimetric assays where appropriate (see
Titrimetry  541
541
).
Tolerances—
The limits specified in the monographs for Pharmacopeial articles are established with a view to the use of these articles as drugs, nutritional or dietary supplements, or devices, except where it is indicated otherwise. The use of the molecular formula for the active ingredient(s) named in defining the required strength of a Pharmacopeial article is intended to designate the chemical entity or entities, as given in the complete chemical name of the article, having absolute (100 percent) purity.
A dosage form shall be formulated with the intent to provide 100 percent of the quantity of each ingredient declared on the label. The tolerances and limits stated in the Definitions in the monographs for Pharmacopeial articles allow for analytical error, for unavoidable variations in manufacturing and compounding, and for deterioration to an extent considered acceptable under practical conditions. Where the minimum amount of a substance present in a nutritional or dietary supplement is required to be higher than the lower tolerance limit allowed for in the monograph because of applicable legal requirements, then the upper tolerance limit contained in the monograph shall be increased by a corresponding amount.
The specified tolerances are based upon such attributes of quality as might be expected to characterize an article produced from suitable raw materials under recognized principles of good manufacturing practice.
The existence of compendial limits or tolerances does not constitute a basis for a claim that an official substance that more nearly approaches 100 percent purity “exceeds” the Pharmacopeial quality. Similarly, the fact that an article has been prepared to closer tolerances than those specified in the monograph does not constitute a basis for a claim that the article “exceeds” the Pharmacopeial requirements.
Interpretation of Requirements—
Analytical results observed in the laboratory (or calculated from experimental measurements) are compared with stated limits to determine whether there is conformance with compendial assay or test requirements. The observed or calculated values usually will contain more significant figures than there are in the stated limit, and a reportable result is to be rounded off to the number of places that is in agreement with the limit expression by the following procedure. Intermediate calculations (e.g., slope for linearity in
Validation of Compendial Methods  1225
1225
) may be rounded for reporting purposes, but the original value (not rounded) should be used for any additional required calculations. Rounding off should not be done until the final calculations for the reportable value have been completed.
[NOTE—Limits, which are fixed numbers, are not rounded off.
]
A reportable value is often a summary value for several individual determinations. It is the end result of a completed measurement method, as documented. It is the value compared with the acceptance criterion. In most cases, the reportable value is used as documentation for internal or external users.
When rounding off is required, consider only one digit in the decimal place to the right of the last place in the limit expression. If this digit is smaller than 5, it is eliminated and the preceding digit is unchanged. If this digit is greater than 5, it is eliminated and the preceding digit is increased by one. If this digit equals 5, the 5 is eliminated and the preceding digit is increased by one.
Illustration of Rounding Numerical Values for Comparison
with Requirements |
Compendial
Requirement |
Unrounded
Value |
Rounded
Result |
Conforms |
Assay limit 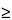 98.0% 98.0% |
97.96% |
98.0% |
Yes |
|
97.92% |
97.9% |
No |
|
97.95% |
98.0% |
Yes |
Assay limit 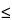 101.5% 101.5% |
101.55% |
101.6% |
No |
|
101.46% |
101.5% |
Yes |
|
101.45% |
101.5% |
Yes |
Limit test 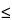 0.02% 0.02% |
0.025% |
0.03% |
No |
|
0.015% |
0.02% |
Yes |
|
0.027% |
0.03% |
No |
Limit test 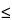 3 ppm 3 ppm |
0.00035% |
0.0004% |
No |
|
0.00025% |
0.0003% |
Yes |
|
0.00028% |
0.0003% |
Yes |