Packaging and storage—
Preserve in tight, light-resistant containers.
Identification—
B:
It responds to the tests for
Bismuth  191
191
.
pH  791
791 :
:
between 2.7 and 5.0, in a solution prepared as follows. Mix 10 g of Bismuth Subsalicylate and 90 mL of water, shake by mechanical means for 10 minutes, and filter.
Limit of nitrate—
To 0.1 g of it add 10 mL of water, carefully add 20 mL of sulfuric acid, and mix. The resulting solution should not be more yellow than a reference solution concomitantly prepared by adding to 0.1 g of salicylic acid, 6 mL of water, 4.0 mL of a solution containing 100 µg of nitrate (NO3) per mL, and 20 mL of sulfuric acid, and mixing (0.4%).
Arsenic, Method I  211
211 —
—
Prepare the
Test Preparation as follows. Triturate about 300 mg of it, accurately weighed, with an equal weight of calcium hydroxide, and ignite. Dissolve the residue in 5 mL of 3 N hydrochloric acid. The limit is 10 µg per g.
Limit of free salicylic acid—
Mobile phase—
Prepare a mixture of methanol and 0.06
M acetic acid (550:450), filter, and degas. Make adjustments if necessary (see
System Suitability under
Chromatography  621
621
).
Diluent—
Use a mixture of acetonitrile and water (1:1).
Standard solution—
Transfer about 20 mg of
USP Salicylic Acid RS, accurately weighed, to a 100-mL volumetric flask, add 20 mL of
Diluent, and swirl to dissolve. Dilute with
Diluent to volume, and mix. Transfer 5.0 mL of this stock solution to a 50-mL volumetric flask, dilute with
Diluent to volume, and mix. This
Standard solution contains about 0.02 mg of
USP Salicylic Acid RS per mL.
Test solution—
Add about 260 mg of Bismuth Subsalicylate, accurately weighed, to a glass centrifuge tube, add about 12 mL of acetonitrile, shake by mechanical means for 20 minutes, and centrifuge. Decant the supernatant into a suitable container. Repeat the acetonitrile addition, shaking, centrifuging, and decanting, combining the decanted liquid with the first decantate. Pass the combined liquid through a filter having a 0.5-µm or finer porosity, collecting the filtrate in a 50-mL volumetric flask. Wash the container with 5 mL of acetonitrile, and filter the wash, collecting the filtrate in the volumetric flask. Dilute with water to volume, and mix.
Chromatographic system—
The liquid chromatograph is equipped with a 300-nm detector, a 3.2-mm × 1.5-cm guard column that contains 5-µm packing L1, and a 4.6-mm × 30-cm analytical column that contains 5-µm packing L1. The flow rate is about 1 mL per minute. Chromatograph the Standard solution, and record the peak responses as directed for Procedure: the tailing factor is not more than 2.0; and the relative standard deviation for replicate injections is not more than 2.0%.
Procedure—
Separately inject equal volumes (about 20 µL) of the
Standard solution and the
Test solution into the chromatograph, and measure the peak area responses. Calculate the percentage of free salicylic acid in the Bismuth Subsalicylate taken by the formula:
5000(C / W)(rU / rS),
in which
C is the concentration, in mg per mL, of
USP Salicylic Acid RS in the
Standard solution; W is the weight, in mg, of the Bismuth Subsalicylate taken to prepare the
Test solution; and
rU and
rS are the salicylic acid peak area responses obtained from the
Test solution and the
Standard solution, respectively. Not more than 0.2% is found.
Limit of copper, lead, and silver—
Standard solution—
Transfer 3.0 mL each of solutions containing 1000 µg per mL of copper, lead, and silver, respectively, to a 2000-mL volumetric flask, dilute with 1 M nitric acid to volume, and mix. [NOTE—The concentrations of copper, lead, and silver may be modified by using different volumes or concentrations to bring the absorption response within the working range of the atomic absorption spectrophotometer.]
Test solution—
Ignite about 3 g of it, accurately weighed, in a porcelain crucible, cool, and cautiously add 6 M nitric acid to dissolve the residue, and evaporate on a steam bath. Ignite the residue, cool, and transfer the residue to a tared conical flask, wash the flask with about 5 mL of 6 M nitric acid, adding the wash to the conical flask. Dissolve the residue with the aid of heat, and add water to obtain a solution weighing 20.0 g.
[NOTE—The concentrate of Bismuth Subsalicyclate may be modified by using the same proportions used for modifying the Standard solution by using a different quantity or by further dilution.]
Procedure—
Concomitantly determine the absorbances of the
Standard solution and the
Test solution at the emission lines of 324.7 nm, 217 nm, and 328.1 nm, for copper, lead, and silver, respectively, with an atomic absorption spectrophotometer (see
Spectrophotometry and Light-scattering  851
851
) equipped with copper, lead, and silver hollow-cathode lamps and an oxidizing flame. The absorbances of the
Test solution do not exceed those of the
Standard solution for each element (10 µg per g).
Limit of soluble bismuth—
Standard solution—
Transfer 242.0 mg of bismuth nitrate pentahydrate to a 100-mL volumetric flask, add 3 mL of 1.5 M nitric acid and swirl to dissolve, dilute with water to volume, and mix. Transfer 1.0 mL of this solution to a 500-mL volumetric flask, add 250 mL of 1.5 M nitric acid, dilute with water to volume, and mix. This solution contains 2 µg of bismuth (Bi) per mL. [NOTE—The concentration of bismuth in this solution may be modified by using a lesser dilution or by further dilution to bring the absorption response within the working range of the atomic absorption spectrophotometer.]
Test solution—
Prepare a mixture of 5.0 g of Bismuth Subsalicylate and 100 mL of water, and stir the suspension thus obtained for 2 hours at 20

to 23

. Filter through filter paper. Filter the filtrate thus obtained through a filter having a porosity of 0.1 µm or less. To 10.0 mL of the filtrate add 0.1 mL of nitric acid.
[NOTE—The concentrate of Bismuth Subsalicyclate may be modified by using the same proportions used for modifying the
Standard Solution by using a different quantity or by further dilution.
]
Procedure—
Concomitantly determine the absorbances of the
Standard solution and the
Test solution at the emission line of 223.06 nm for bismuth with an atomic absorption spectrophotometer (see
Spectrophotometry and Light-scattering  851
851
) equipped with a bismuth hollow-cathode lamp and an oxidizing flame. The absorbances of the
Test solution do not exceed those of the
Standard solution (40 µg per g).
Assay for bismuth—
Transfer about 300 mg of Bismuth Subsalicylate, previously dried at 105

for 3 hours and accurately weighed, to a porcelain crucible, and ignite. Allow it to cool, and add about 2 mL of nitric acid to the residue, dropwise, warming until complete solution has been effected. Add about 60 mL of water and 0.3 mL of
xylenol orange TS, and titrate with 0.05
M edetate disodium VS to a yellow endpoint. Each mL of 0.05
M edetate disodium is equivalent to 10.45 mg of bismuth (Bi).
Assay for total salicylates—
Ferric ammonium sulfate solution—
Transfer 20.0 mL of
ferric ammonium sulfate TS and 5.0 mL of 1 N hydrochloric acid to a 100-mL volumetric flask, dilute with water to volume, and mix.
Standard stock solution—
Prepare a solution of
USP Salicylic Acid RS in water having a known concentration of about 0.2 mg per mL.
Standard preparation—
Transfer 25.0 mL of the Standard stock solution to a beaker, add about 70 mL of water, adjust with 0.5 N sodium hydroxide or 1 N hydrochloric acid to a pH of 4.5. Transfer this solution to a 100-mL volumetric flask with the aid of water, dilute with water to volume, and mix.
Assay preparation—
Transfer about 52 mg of Bismuth Subsalicylate, previously dried at 105

for 3 hours and accurately weighed, to a 200-mL volumetric flask. Add 10 mL of 0.5 N sodium hydroxide, heat on a steam bath for 15 minutes, allow to cool, dilute with water to volume, and mix. Centrifuge about 70 mL of this solution, then transfer 50.0 mL of the clear supernatant to a beaker. Add about 40 mL of water, and adjust with 0.5 N sodium hydroxide or 1 N hydrochloric acid to a pH of 4.5. Transfer this solution to a 100-mL volumetric flask with the aid of water, dilute with water to volume, and mix.
Blank—
Use water previously adjusted with 0.5 N sodium hydroxide or 1 N hydrochloric acid to a pH of 4.5.
Procedure—
To three separate 50-mL conical flasks add 25.0 mL of the
Standard preparation, the
Assay preparation, and the
Blank, respectively. To each flask add 1.0 mL of
Ferric ammonium sulfate solution, and mix to produce the
Reacted standard preparation, the
Reacted assay preparation, and the
Reacted blank solution, respectively. To a second set of three separate 50-mL conical flasks add 25.0 mL of the
Standard preparation, the
Assay preparation, and the
Blank, respectively. To each flask add 1.0 mL of 0.05 N hydrochloric acid, and mix to produce the
Unreacted standard preparation, the
Unreacted assay preparation, and the
Unreacted blank solution, respectively. Concomitantly determine the absorbances of the six solutions at the wavelength of maximum absorption at about 525 nm, using water to zero the spectrophotometer. Calculate the percentage of total salicylates in the portion of Bismuth Subsalicylate taken by the formula:
10,000(
C / W)[(
AUr 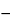 AUu
AUu 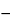 B
B) / (
ASr 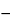 ASu
ASu 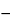 B
B)],
in which
C is the concentration, in mg per mL, of
USP Salicylic Acid RS in the
Standard stock solution; W is the weight, in mg, of Bismuth Subsalicylate taken to prepare the
Assay preparation; AUr is the absorbance of the
Reacted assay preparation; AUu is the absorbance of the
Unreacted assay preparation; ASr is the absorbance of the
Reacted standard preparation; ASu is the absorbance of the
Unreacted standard preparation; and
B is the difference in the absorption of the
Reacted blank solution and the absorption of the
Unreacted blank solution.