Packaging and storage—
Preserve in tight, light-resistant containers. Store at 25

, excursions permitted between 15

and 30

.
Assay—
Mobile phase—
Prepare a filtered and degassed solution containing a mixture of water, acetonitrile, and triethylamine (700:300:9). Make adjustments if necessary (see
System Suitability under
Chromatography  621
621
).
Internal standard solution—
Transfer about 113 mg of papaverine hydrochloride to a 1-L flask. Add a mixture of 0.01 M tartaric acid and acetonitrile (2:1) to volume, and mix.
Standard preparation—
Transfer about 33 mg of
USP Ergoloid Mesylates RS, accurately weighed, to a 100-mL volumetric flask. Dissolve in and dilute with
Internal standard solution to volume, and mix. Use a freshly prepared solution.
Assay preparation—
Weigh and finely powder not fewer than 20 Sublingual Tablets. Transfer an accurately weighed portion of the powder, equivalent to about 5 mg of ergoloid mesylates, to a 50-mL centrifuge tube. Add 15.0 mL of Internal standard solution, insert the stopper into the tube, and shake for about 15 minutes. Centrifuge, filter if necessary, and use the clear supernatant.
Chromatographic system (see Chromatography  621
621 )—
)—
The liquid chromatograph is equipped with a 280-nm detector and a 4.6-mm × 15-cm column that contains packing L1.
[NOTE—Use an L1 column capable of handling pH values greater than 11.
] The flow rate is about 1.5 mL per minute. Chromatograph the
Standard preparation, and record the peak responses as directed for
Procedure: the column efficiency determined for the dihydro-
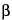
-ergocryptine mesylate peak is not less than 1000 theoretical plates; the tailing factor for the dihydro-
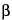
-ergocryptine mesylate peak is not more than 2.0; the resolution,
R, between the dihydro-
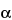
-ergocryptine mesylate and dihydroergocristine mesylate peaks is not less than 2.0, and between dihydroergocristine and dihydro-
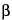
-ergocryptine peaks is not less than 2.0; and the relative standard deviation of the ratio of the sum of the four peaks to the internal standard for replicate injections is not more than 1.5%.
Procedure—
Separately inject equal volumes (about 20 µL) of the
Standard preparation and the
Assay preparation into the chromatograph, record the chromatograms, and measure the responses for the major peaks. The order of elution is papaverine, dihydroergocornine, dihydro-
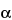
-ergocryptine, dihydroergocristine, and dihydro-
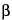
-ergocryptine. Calculate the quantity, in mg, of ergoloid mesylates in the portion of Sublingual Tablets taken by the formula:
15C(RU / RS),
in which
C is the concentration, in mg per mL, of
USP Ergoloid Mesylates RS in the
Standard preparation; and
RU and
RS are the sums of the ratios of responses of the four major peaks to the response of the internal standard peak obtained from the
Assay preparation and the
Standard preparation, respectively. Calculate the percentage of each of the individual alkaloids taken by the formula:
100Ri(MW)i /S[Ri(MW)i],
in which
Ri is the peak response ratio of the individual alkaloid to the internal standard; (
MW)
i is the molecular weight of the individual alkaloid; and
S[
Ri(
MW)
i] is the summation of the products of peak response ratios and molecular weights for the four alkaloids.