(C
18H
37N
5O
9)
2·5H
2SO
4





1425.45
D-Streptamine,
O-3-amino-3-deoxy-
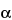
-
D-glucopyranosyl-(1
®6)-
O-[2,6-diamino-2,3,6-tridoexy-
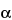
-
D-ribo-hexopyranosyl-(1
®4)]-2-deoxy-, sulfate (2:5) (salt).
O-3-Amino-3-deoxy-
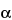
-
D-glucopyranosyl-(1
®4)-
O-[2,6-diamino-2,3,6-trideoxy-
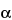
-
D-ribo-hexopyranosyl-(1
®6)]-2-deoxy-
L-streptamine, sulfate (2:5) (salt)



[
79645-27-5].
Packaging and storage—
Preserve in tight containers.
Labeling—
Where it is intended for use in preparing injectable dosage forms, the label states that it is sterile or must be subjected to further processing during the preparation of injectable dosage forms.
Identification—
A:
It responds to the Identification tests under Tobramycin.
B:
It responds to the tests for
Sulfate  191
191
.
pH  791
791 :
:
between 6.0 and 8.0, in a solution containing 40 mg per mL.
Other requirements—
It meets the requirements for
Residue on ignition,
Heavy metals, and
Chromatographic purity under
Tobramycin. Where the label states that Tobramycin Sulfate is sterile, it meets the requirements for
Sterility Tests  71
71
and for
Bacterial endotoxins under
Tobramycin for Injection. Where the label states that Tobramycin Sulfate must be subjected to further processing during the preparation of injectable dosage forms, it meets the requirements for
Bacterial endotoxins under
Tobramycin for Injection.
Assay—
Mobile phase
,
2,
4-Dinitrofluorobenzene reagent,
Tris(
hydroxymethyl)
aminomethane reagent,
Standard preparation, Derivatization procedure,
Resolution solution, and
Chromatographic system—Proceed as directed in the
Assay under
Tobramycin.
Assay preparation—
Transfer an accurately weighed quantity of Tobramycin Sulfate, equivalent to about 50 mg of tobramycin (C18H37N5O9), to a 250-mL volumetric flask, dissolve in and dilute with water to volume, and mix.
Procedure—
Proceed as directed for
Procedure in the
Assay under
Tobramycin. Calculate the quantity, in µg, of tobramycin (C
18H
37N
5O
9) in each mg of the Tobramycin Sulfate taken by the formula:
250(CE / W)(rU / rS),
in which the terms are as defined therein.