C
28H
47NO
4S·C
4H
4O
4





609.82
Acetic acid, [[2-(diethylaminoethyl]thio]-, 6-ethenyl-decahydro-5-hydroxy-4,6,9,10-tetramethyl-1-oxo-3a,9-propano-3a
H-cyclopentacycloocten-8-yl ester [3a
S-(3a
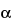
,4
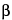
,5
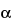
,6
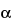
,8
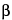
,9
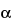
,9a
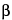
,10
S*)]-, (
E)-2-butenedioate (1:1) (salt).
[[2-(Diethylamino)ethyl]thio]acetic acid 8-ester with (3a
S,4
R,5
S,6
S,8
R,9
R,9a
R,10
R)-octahydro-5,8-dihydroxy-4,6,9,10-tetramethyl-6-vinyl-3a,9-propano-3a
H-cyclopentacycloocten-1(4
H)-one fumarate (1:1) (salt)



[
55297-96-6].
Packaging and storage—
Preserve in tight, light-resistant containers, and store at room temperature.
Labeling—
Label it to indicate that it is for veterinary use only.
Color and clarity of solution—
Transfer about 5.0 g of Tiamulin Fumarate to a 100-mL volumetric flask, and dissolve in and dilute with water to volume: the solution is clear and colorless, and the absorbance of the solution, determined in a 1-cm cell at 400 and 650 nm, is not greater than 0.150 and 0.030 absorbance units, respectively.
Identification—
B:
The retention time of the tiamulin fumarate peak in the chromatogram of the Assay preparation corresponds to that in the chromatogram of the Standard preparation, as obtained in the Assay.
Specific rotation  781S
781S :
:
between +24

and +28

, on the dried basis, measured at 20

.
Test solution:
5.0 mg per mL, in dioxane.
pH  791
791 :
:
between 3.1 and 4.1.
Test solution:
1.0 g per 100 mL of water.
Loss on drying  731
731 —
—
Dry it in vacuum at 105

for 3 hours: it loses not more than 0.5% of its weight.
Limit of residual solvents—
Internal standard solution—
Dilute 0.3 mL of n-butanol to 1000 mL.
Standard solution—
Transfer about 500 mg each of acetone, ethyl acetate, and isobutyl acetate, each accurately weighed, to a 250-mL volumetric flask, dilute with the Internal standard solution to volume, and mix. Transfer 2.5 mL of the solution so obtained to a 20-mL volumetric flask, dilute with the Internal standard solution to volume, and mix.
Test solution—
Transfer about 1 g of Tiamulin Fumarate, accurately weighed, to a 20-mL volumetric flask, dissolve in and dilute with the Internal standard solution to volume, and mix.
Chromatographic system (see Chromatography  621
621 )—
)—
The gas chromatograph is equipped with a flame-ionization detector and a 0.25-mm × 30-m capillary column coated with a 0.5-µm film of phase G16. The carrier gas is helium, flowing at a rate of 1.07 mL per minute, and the split flow ratio is 50:1. The column temperature is maintained at 75

, the injection port temperature is maintained at 250

, and the detector temperature is maintained at 300

. Chromatograph the
Standard solution, and record the peak responses as directed for
Procedure: the relative retention times are about 0.34, 0.38, 0.57, and 1.0 for acetone, ethyl acetate, isobutyl acetate, and
n-butanol, respectively; the resolution,
R, between acetone and ethyl acetate is not less than 2.0; the tailing factor for each of the analyte peaks is not more than 2; and the relative standard deviation for replicate injections is not more than 2%.
Procedure—
Separately inject equal volumes (about 1.0 µL) of the
Standard solution and the
Test solution into the chromatograph, record the chromatograms, and measure the peak responses for acetone, ethyl acetate, isobutyl acetate, and
n-butanol. Calculate the percentages of acetone, ethyl acetate, and isobutyl acetate in the portion of Tiamulin Fumarate taken by the formula:
1.25(WS / WU)(RU / RS),
in which
WS is the weight, in mg, of the solvent of interest taken to prepare the
Standard solution; WU is the weight, in mg, of Tiamulin Fumarate taken to prepare the
Test solution; and
RU and
RS are the peak response ratios of the solvent of interest to the internal standard obtained from the
Test solution and the
Standard solution, respectively: not more than 0.5% each of acetone, ethyl acetate, and isobutyl acetate is found; and the sum of the percentages of acetone, ethyl acetate, and isobutyl acetate is not more than 0.5%.
Chromatographic purity—
Dilute perchloric acid solution, Buffer solution, Mobile phase, System suitability solution, and Chromatographic system—
Proceed as directed in the Assay.
Standard solution—
Use the Standard preparation prepared as directed in the Assay.
Test solution—
Use the Assay preparation prepared as directed in the Assay.
Procedure—
Separately inject equal volumes (about 20 µL) of the
Standard solution and the
Test solution into the chromatograph, record the chromatogram, identify the tiamulin fumarate peak, and measure all the peak responses.
[NOTE—Possible tiamulin fumarate impurities include, but are not limited to, pleuromutilin, mutilin, 14-acetyl mutilin, 11-monoacetyl mutilin, tiamulin related compound A, 11,14-diacetyl mutilin, 8-dimethylderivative, bisdimethylthioderivative, and 11-ketoderivative, their retention times, relative to tiamulin fumarate, being about 0.25, 0.3, 0.5, 0.6, 0.8, 1.1, 1.3, 1.4, and 2.3, respectively.
] Calculate the area percentage of each impurity, relative to tiamulin fumarate, in the portion of Tiamulin Fumarate taken by the formula:
100(ri / rU),
in which
ri and
rU are the peak responses of each impurity and tiamulin fumarate, respectively: not more than 1.0% of any identified impurity is found; not more than 0.5% of any unidentified impurity is found; and not more than 2.0% of total impurities is found.
Content of fumarate—
Dissolve about 450 mg of Tiamulin Fumarate, accurately weighed, in 60 mL of a mixture of alcohol and water (1:1). Titrate with 0.1 N sodium hydroxide VS, determining the endpoint potentiometrically, using a glass–calomel electrode (see
Titrimetry  541
541
). Perform a blank determination, and make any necessary correction. Each mL of 0.1 N sodium hydroxide is equivalent to 5.8 mg of fumarate: between 83.7 and 87.3 mg of fumarate is found.
Assay—
Dilute perchloric acid solution—
Prepare a solution containing 6% of perchloric acid.
Buffer solution—
Transfer 10 g of ammonium carbonate to a 1000-mL volumetric flask, and dissolve in about 800 mL of water. Add 24 mL of Dilute perchloric acid solution, dilute with water to volume, mix, and filter.
Mobile phase—
Prepare a mixture of methanol, Buffer solution, and acetonitrile, (49:28:23), filter, and degas.
Standard preparation—
Dissolve an accurately weighed quantity of
USP Tiamulin Fumarate RS in
Mobile phase to obtain a solution having a known concentration of about 4 mg per mL.
Assay preparation—
Transfer about 200 mg, accurately weighed, to a 50-mL volumetric flask, dissolve in and dilute with Mobile phase to volume, and mix.
Chromatographic system (see Chromatography  621
621 )—
)—
The liquid chromatograph is equipped with a 212-nm detector and a 4.6-mm × 25-cm column that contains 5-µm packing L1. The flow rate is about 1.2 mL per minute. The column temperature is maintained at 30 ± 3

. Chromatograph the
Standard preparation and the
System suitability solution, and record the peak responses as directed for
Procedure: the tiamulin related compound A peak elutes prior to the tiamulin fumarate peak; the resolution,
R, between tiamulin related compound A and tiamulin fumarate is not less than 2.0; the capacity factor,
k¢, determined from the tiamulin fumarate peak, is not less than 2.0; the column efficiency is not less than 14,000 theoretical plates; the tailing factor is not more than 2.0; and the relative standard deviation for replicate injections is not more than 2.0%.
Procedure—
Separately inject equal volumes (about 20 µL) of the
Standard preparation and the
Assay preparation into the chromatograph, record the chromatograms, and measure the responses for the major peaks. Calculate the quantity, in mg, of C
28H
47NO
4S·C
4H
4O
4 in the portion of Tiamulin Fumarate taken by the formula:
50C(rU / rS),
in which
C is the concentration, in mg per mL, of
USP Tiamulin Fumarate RS in the
Standard preparation; and
rU and
rS are the tiamulin fumarate peak responses obtained from the
Assay preparation and the
Standard preparation, respectively.