Procedure—
Weigh and finely powder not less than 20 Tablets. Transfer an accurately weighed portion of the powder, equivalent to about 0.7 mg of norethindrone, to a 50-mL volumetric flask, and add anhydrous methanol to volume. Mix, and allow to stand for 10 minutes, with occasional mixing. Filter a portion of the mixture to clarify the solution, and transfer 10.0 mL of the filtrate to a suitable container. Add 2.0 mL of
Isoniazid reagent, mix, seal, and allow to stand for 30 minutes. This is the
Assay preparation. Transfer a second 10.0-mL portion of the filtrate to a suitable container, add 2.0 mL of methanol, and mix. This is the
Assay blank preparation. Transfer 10.0 mL of methanol to a suitable container, add 2.0 mL of
Isoniazid reagent, mix, seal, and allow to stand for 30 minutes. This is the
Reagent blank preparation. Prepare a
Standard preparation by transferring 10.0 mL of a solution of
USP Norethindrone RS in methanol having a concentration of about 14 µg per mL to a suitable container. Add 2.0 mL of
Isoniazid reagent, mix, seal, and allow to stand for 30 minutes. Concomitantly determine the absorbances of these solutions in 1-cm cells, at about 380 nm, with a suitable spectrophotometer, using methanol as the reference for the
Assay blank preparation, and using the
Reagent blank preparation as the reference for the
Assay preparation and the
Standard preparation. Calculate the quantity, in mg, of C
20H
26O
2 in the portion of Tablets taken by the formula:
0.05
C(
AU 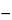 AB
AB) /
AS,
in which
C is the concentration, in µg per mL, of the
Standard preparation, and
AU,
AB, and
AS are the absorbances of the
Assay preparation, the
Assay blank preparation, and the
Standard preparation, respectively.