C
17H
17NO
2·HCl·½H
2O





312.79
4
H-Dibenzo[
de,
g]quinoline-10,11-diol, 5,6,6a,7-tetrahydro-6-methyl-, hydrochloride, hemihydrate, (
R)-.
6a
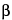
-Aporphine-10,11-diol hydrochloride hemihydrate



[
41372-20-7].
Anhydrous
303.79



[
314-19-2].
Packaging and storage—
Preserve in small, tight, light-resistant containers. Containers from which Apomorphine Hydrochloride is to be taken for immediate use in compounding prescriptions contain not more than 350 mg.
Color of solution—
Place 100 mg in a suitable test tube, add 10 mL of cold, oxygen-free water, and agitate gently until dissolved: the color of the resulting solution, observed promptly after the Apomorphine Hydrochloride has dissolved, is not more intense than that of a color standard prepared as follows. Dissolve 5 mg of Apomorphine Hydrochloride in 100.0 mL of water. Transfer 1.0 mL of this solution to a test tube of the same size as that used for the test solution, dilute with 6 mL of water, add 1 mL of sodium bicarbonate solution (1 in 20), and then add 0.50 mL of iodine TS. Allow to stand for 30 seconds, add 0.60 mL of sodium thiosulfate solution (1 in 40), and dilute with water to 10 mL.
Identification—
B:
To 5 mL of a solution (1 in 100) add a slight excess of sodium bicarbonate solution (1 in 20): a white or greenish-white precipitate is formed. Add 3 drops of iodine TS, and shake vigorously: an emerald-green color is produced. Add 5 mL of ether and, after vigorous shaking, allow the layers to separate: the ether is colored deep ruby-red while the water layer retains its green color.
C:
Dissolve it in nitric acid: a dark purple solution is produced.
D:
To a solution of it add silver nitrate TS: a white precipitate, which is insoluble in nitric acid, is formed. This precipitate soon darkens by reduction to metallic silver, the reduction being accelerated by the addition of 6 N ammonium hydroxide.
Specific rotation  781S
781S :
:
between
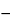
60.5

and
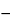
63.0

.
Test solution:
15 mg per mL, in dimethylsulfoxide.
Loss on drying  731
731 —
—
Dry it at 105

for 2 hours: it loses between 2.0% and 3.5% of its weight.
Decomposition products—
Shake 100 mg with 5 mL of ether: the latter acquires not more than a pale reddish color.
Ordinary impurities  466
466 —
—
Solvent for Test Solution:
methanol.
Solvent for Standard Solution:
methanol.
Eluant:
a mixture of 1-butanol, water, and formic acid (7:2:1).
Visualization:
a freshly prepared mixture of 10% ferric chloride solution and 5% potassium ferricyanide solution (2:1). Allow the chromatograms to develop until the solvent front has moved about 8 cm. [NOTE—The development time is 1.5 to 2 hours.] Allow the plates to dry at room temperature for 1 hour prior to spraying.
Assay—
Dissolve about 300 mg of Apomorphine Hydrochloride, accurately weighed, in 100 mL of glacial acetic acid with the aid of heat provided by a steam bath. Add 0.1 mL of acetic anhydride to the hot solution, and stir for 5 minutes. Cool to room temperature, add 5 mL of
mercuric acetate TS and 0.25 mL of
crystal violet TS, and titrate with 0.1 N perchloric acid VS to a blue endpoint. Perform a blank determination, and make any necessary correction. Each mL of 0.1 N perchloric acid is equivalent to 30.38 mg of C
17H
17NO
2·HCl.