Identification—
Macerate a quantity of powdered Tablets, equivalent to about 50 mg of amphetamine sulfate, with 10 mL of water for 30 minutes, and filter into a small flask. To the filtrate add 3 mL of 1 N sodium hydroxide. Cool to about 10

to 15

, add 1 mL of a mixture of 1 volume of benzoyl chloride and 2 volumes of absolute ether, insert the stopper, and shake well for 3 minutes. Filter the precipitate, wash with about 15 mL of cold water, and recrystallize twice from diluted alcohol: the crystals of the benzoyl derivative of amphetamine so obtained, after drying at 80

for 2 hours, melt between 131

and 135

, the procedure for
Class I being used (see
Melting Range or Temperature  741
741
).
Dissolution, Procedure for a Pooled Sample  711
711 —
—
Medium:
water; 500 mL.
Apparatus 1:
100 rpm.
Time:
45 minutes.
Mobile phase—
Dissolve 1.1 g of sodium 1-heptanesulfonate in 575 mL of water. Add 25 mL of dilute glacial acetic acid (14 in 100) and 400 mL of methanol. Adjust by the dropwise addition of glacial acetic acid to a pH of 3.3 ± 0.1, if necessary, filter, and degas the solution. Make adjustments if necessary (see
System Suitability under
Chromatography  621
621
).
Chromatographic system
(see
Chromatography  621
621
)—The liquid chromatograph is equipped with a 254-nm detector and a 3.9-mm × 30-cm column that contains packing L1. The flow rate is about 1 mL per minute. Chromatograph replicate injections of the Standard solution, and record the peak responses as directed for
Procedure: the relative standard deviation is not more than 2.0%.
Procedure—
Inject a volume (about 500 µL) of a filtered portion of the solution under test into the chromatograph, record the chromatogram, and measure the response for the major peak. Calculate the quantity of (C
9H
13N)
2·H
2SO
4 dissolved in comparison with a Standard solution having a known concentration of
USP Dextroamphetamine Sulfate RS in the same medium and similarly chromatographed.
Tolerances—
Not less than 75% (Q) of the labeled amount of (C9H13N)2·H2SO4 is dissolved in 45 minutes.
Assay—
Assay preparation—
Weigh and finely powder not fewer than 20 Tablets. Transfer an accurately weighed portion of the powder, equivalent to about 5 mg of amphetamine sulfate, to a 100-mL beaker, add 2 mL of hydrochloric acid solution (1 in 100), swirl gently to wet the powder thoroughly, warm on a steam bath for about 1 minute, with occasional gentle swirling, and cool. Add 3 g of purified siliceous earth, and mix until a fluffy mixture is obtained.
Procedure—
Proceed as directed under
Amphetamine Assay  331
331
. Calculate the quantity, in mg, of (C
9H
13N)
2·H
2SO
4 in the portion of Tablets taken by the formula:
0.01
C[(
AU257 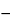 AU280
AU280) / (
AS257 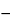 AS280
AS280)],
in which
C is the concentration, in µg per mL, of
USP Dextroamphetamine Sulfate RS in the
Standard preparation.