Benzenepropanoic acid, 3-amino-
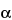
-ethyl-2,4,6-triiodo-, (±)-.
(±)-3-Amino-
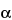
-ethyl-2,4,6-triiodohydrocinnamic acid



[
96-83-3].
Packaging and storage—
Preserve in tight, light-resistant containers.
Identification—
Mix about 100 mg with 500 mg of sodium carbonate in a crucible, and heat until thoroughly charred. Cool, add 5 mL of hot water, heat on a steam bath for 5 minutes, and filter: the solution responds to the tests for
Iodide  191
191
.
Free iodine—
Shake about 200 mg with 2 mL of water and 2 mL of chloroform for 1 minute: the chloroform layer shows no violet color.
Halide ions—
Place about 500 mg in a glass-stoppered, 50-mL cylinder, add 10 mL of 2 N nitric acid and 15 mL of water, shake for 5 minutes, and filter through paper: 10 mL of the filtrate shows no greater turbidity than corresponds to 0.05 mL of 0.020 N hydrochloric acid (see
Chloride and Sulfate  221
221
).
Heavy metals, Method II  231
231 :
:
0.002%.
Assay—
Transfer about 250 mg of Iopanoic Acid, accurately weighed, to a glass-stoppered, 250-mL conical flask. Add 30 mL of 1.25 N sodium hydroxide and 500 mg of powdered zinc, and reflux the mixture for 30 minutes. Cool to room temperature, wash the condenser with 20 mL of water, and filter the mixture. Wash the flask and the filter with small portions of water, adding the washings to the filtrate. Add to the filtrate 5 mL of glacial acetic acid and 1 mL of
tetrabromophenolphthalein ethyl ester TS, and titrate with 0.05 N silver nitrate VS until the color of the yellow precipitate just changes to green. Each mL of 0.05 N silver nitrate is equivalent to 9.516 mg of C
11H
12I
3NO
2.