Botanic characteristics—
Macroscopic—
Subglobular compound bulbs, 3 to 5 cm in width, consisting of 8 to 20 cloves, the whole surrounded by 2 to 5 layers of white scale leaves attached to a flattened, circular base; cloves ovoid and 3- to 4-sided, summit acute, narrowed into a threadlike portion of fiber base, truncate, each clove covered with a white scale leaf and a pinkish white epidermis, easily separated from the solid portion, consisting of two flaky scale leaves and two yellowish green conduplicate foliage leaves.
Microscopic—
The protective leaf contains an epidermis enclosing a mesophyll free from chlorophyll. The outer epidermis consists of lignified sclereid cells of thick, pitted walls, elongated, covered with thin cuticle, long fibers up to 500 µm in length and 30 µm in width.
The cortical cells are thick-walled, nonlignified, tending to collapse on maturity, isodiametric, and contain purple pigments. The vascular bundles consist of lignified spiral and annular vessels. The storage leaves show an outer epidermis of thin, delicate cells of variable shape, arranged in somewhat irregular rows, 60 µm in length and 30 µm in width. Stomata are present on the outer epidermis only at the extreme tip near the base of the foliage leaves.
The mesophyll consists of swollen storage parenchyma cells filled with fine granular reserve material; scattered in the cortex are about 20 laticiferous tubes, 500 to 1000 µm in length. Two series of vascular bundles consisting of narrow lignified spiral and annular vessels are arranged in the mesophyll.
Identification—
Test solution—
Cut a freeze-dried garlic bulb into small pieces, transfer about 1 g of the cut pieces to an extractor, and extract with two 20-mL portions of a mixture of methanol and water (1:1), combining the extracts. Concentrate to a small volume (about 5 mL), using a rotary evaporator.
Standard solution A:
0.5 mg of USP L-Methionine RS per mL.
Standard solution B:
0.5 mg of
USP Alliin RS per mL, in a mixture of methanol and water (1:1).
Application volume:
20 µL, applied separately as 10-mm bands.
Developing solvent system:
a mixture of butyl alcohol, n-propyl alcohol, glacial acetic acid, and water (3:1:1:1).
Procedure—
Proceed as directed in the chapter. Spray with a 0.2 in 100 solution of ninhydrin in a mixture of butyl alcohol and 2 N acetic acid (95:5), heat at 100

to 105

for about 10 minutes, and immediately examine the plate. The chromatogram of the
Test solution shows many orange and pinkish violet zones: a violet zone having an
RF value of about 0.89; a pink zone having an
RF value of about 0.5 and corresponding in color and
RF value to that obtained from the chromatogram of
Standard solution A; a pinkish zone having an
RF value of about 0.43; a strong orange zone having an
RF value of about 0.38; a pinkish violet zone having an
RF value of about 0.3 and corresponding in color and
RF value to that obtained from the chromatogram of
Standard solution B; and additional pinkish orange zones situated very close to each other just below the zone attributed to alliin in the chromatogram of
Standard solution B.
B:
Transfer about 10 g of garlic bulbs that have been cut into small pieces to a suitable flask. Add 10 mL of 1 N sodium hydroxide and 10 mL of water, heat the flask in boiling water for 10 minutes, cool, and filter. Add a few drops of freshly prepared
sodium nitroferricyanide TS to 2 mL of the filtrate: appearance of a red or orange-red color indicates the presence of sulfur-containing compounds in the test specimen.
C:
The retention time of the major peak in the chromatogram of the Test solution corresponds to that in the chromatogram of the Standard solution, as obtained in the test for Content of alliin.
Extraction column—
Use a 1-cm × 5-cm solid-phase extraction column that contains styrene-divinylbenzene copolymer packing with a 75- to 150-µm diameter and a 400- to 600-
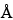
pore size. Condition column prior to use by washing with 50 mL of methanol and with 50 mL of a mixture of water and methanol (7:3).
[NOTE—Do not allow the column to dry.
]
Test solution—
Transfer about 10 g of freshly peeled garlic clove to a 37-mL homogenizing cup, and homogenize with 25 mL of methanol at the highest speed for 1 minute. Centrifuge the mixture, and decant the supernatant to a flask. Add 70 mL of water, and mix. Transfer to Extraction column, allow to drain, and discard to eluate. Wash the column with 50 mL of a mixture of water and methanol (7:3), allow the solvent mixture to drain, and discard the eluate. Finally, elute the crude saponin fraction on the column with 20 mL of methanol, and collect the eluate. Evaporate the solvent to dryness. Dissolve the residue in 4 mL of a mixture of 8% sulfuric acid and alcohol (1:1), transfer the solution to a screw-capped test tube, and heat on a boiling water bath for 5 hours. Cool the test tube, add 20 mL of water, and transfer the solution to a freshly conditioned Extraction column, allow to drain, and discard the eluate. Wash the column with 30 mL of a mixture of methanol and water (7:3), and discard the eluate. Finally, elute the column with 50 mL of methanol. Collect the eluate, evaporate it to dryness, and dissolve the residue in 0.5 mL of methanol.
Developing solvent system:
a mixture of methylene chloride and methanol (15:2).
Application volume:
20 µL, as 7-mm bands.
Spray reagent—
Dissolve 0.5 mL of 4-methoxybenzaldehyde and 0.5 mL of sulfuric acid in alcohol to make 10 mL.
Procedure—
Proceed as directed in the chapter. Spray the plate with
Spray reagent, heat the plate at about 100

to 105

for about 5 minutes, and examine the plate: the chromatogram of the
Test solution exhibits, among several yellowish and grayish green spots, a grayish green spot at an
RF value of about 0.4, corresponding to the grayish green spot due to
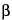
-chlorogenin obtained from the
Standard solution. The chromatogram of the
Test solution exhibits no spot at an
RF value of about 0.2 corresponding to agigenin obtained from the
Standard solution.
Content of alliin—
0.045 M Phosphate buffer—
Dissolve 1.24 g of monobasic sodium phosphate in 100 mL of water, adjust with 0.2 M sodium hydroxide to a pH of 7.1, dilute with water to 200.0 mL, and mix.
0.05 M Phosphate buffer—
Dissolve 1.38 g of monobasic sodium phosphate in 100 mL of water, adjust with 0.2 M sodium hydroxide to a pH of 9.5, dilute with water to 200.0 mL, and mix.
0.01 M Carboxymethoxylamine hemihydrochloride solution—
Dissolve 109 mg of carboxymethoxylamine hemihydrochloride in 100.0 mL of water.
Derivatization reagent—
Dissolve 140 mg of o-phthaldialdehyde in 5 mL of methanol in a 50-mL volumetric flask, add 100 µL of t-butylthiol, dilute with 0.05 M Phosphate buffer to volume, and mix. [NOTE—This reagent may occasionally become opaque during preparation. Store at room temperature, and use within one week.]
Mobile phase—
Prepare a mixture of
0.045 M Phosphate buffer, acetonitrile, 1,4-dioxane, and tetrahydrofuran (69.9:25.0:2.9:2.2). Make adjustments if necessary (see
System Suitability under
Chromatography  621
621
).
Standard solution—
Dissolve an accurately weighed quantity of
USP Alliin RS in a mixture of methanol and water (1:1), and dilute quantitatively, and stepwise if necessary, with a mixture of methanol and water (1:1) to obtain a solution having a known concentration of 0.05 mg per mL. Using a syringe, transfer 0.1 mL of this solution to a septum-capped vial, add 0.5 mL of the
Derivatization reagent, and mix. Allow a reaction time of not less than 2 minutes before injection into the chromatograph.
Test solution—
Transfer about 10.0 g of freshly peeled garlic cloves, accurately weighed, to a 110-mL homogenizing cup. Add 70.0 mL of 0.01 M Carboxymethoxylamine hemihydrochloride solution, and blend at the highest speed for 30 seconds. Centrifuge, and decant the supernatant into a 100-mL volumetric flask. Mix the remaining solids in the cup with 20 mL of 0.01 M Carboxymethoxylamine hemihydrochloride solution, centrifuge, and add the supernatant to the volumetric flask. Dilute the contents of the flask with 0.01 M Carboxymethoxylamine hemihydrochloride solution to volume, and mix. Transfer 10.0 mL of the supernatant homogenate to a 100-mL volumetric flask, dilute with a mixture of methanol and water (1:1) to volume, and mix. Using a syringe, transfer 0.1 mL of this solution to a septum-capped vial, add 0.5 mL of the Derivatization reagent, and mix. Allow a reaction time of not less than 2 minutes before injection into the chromatograph.
Chromatographic system
(see
Chromatography  621
621
)—The liquid chromatograph is equipped with a 337-nm detector and a 4-mm × 10-cm column that contains packing L1. The flow rate is about 1.0 mL per minute.
[NOTE—Alliin exhibits two major peaks representing its diastereomers.
] Chromatograph replicate injections of the
Standard solution, and record the peak areas as directed for
Procedure: the relative standard deviation for replicate injections is not more than 2.0% for each of the major peaks.
Procedure—
Separately inject equal volumes (about 10 µL) of the
Standard solution and the
Test solution into the chromatograph, record the chromatograms, and measure the areas of the responses of the alliin diastereomer peaks. Calculate the percentage of alliin in the portion of Garlic taken by the formula:
100(C / W)(rU / rS),
in which
C is the concentration, in mg per mL, of
USP Alliin RS in the
Standard solution; W is the weight, in g, of garlic cloves taken for the
Test solution; and
rU and
rS are the sums of the peak responses for alliin diastereomers obtained from the
Test solution and the
Standard solution, respectively: not less than 0.5%, calculated on the dried basis, is found.
Content of 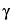 -glutamyl-(S)-allyl-L-cysteine—
-glutamyl-(S)-allyl-L-cysteine—
0.05 M Phosphate buffer—
Dissolve 6.80 g of monobasic potassium phosphate in 900 mL of water, and adjust with phosphoric acid to a pH of 2.6. Dilute with water to 1000.0 mL, and mix.
Mobile phase—
Prepare a mixture of
0.05 M Phosphate buffer and methanol (85:15). Make adjustments if necessary (see
System Suitability under
Chromatography  621
621
).
Standard solution—
Dissolve an accurately weighed quantity of USP
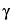
-Glutamyl-(
S)-Allyl-
L-Cysteine RS in a mixture of methanol and water (1:1), and dilute quantitatively, and stepwise if necessary, with a mixture of methanol and water (1:1) to obtain a solution having a known concentration of about 0.08 mg per mL.
Test solution—
Transfer about 10 g of freshly peeled garlic clove, accurately weighed, to a 110-mL homogenizing cup. Add 80 mL of a mixture of methanol and water (1:1), and homogenize at the highest speed for 1 minute. Centrifuge the mixture, and decant the supernatant into a 250-mL volumetric flask. Mix the remaining solids with two 70-mL portions of a mixture of methanol and water (1:1), centrifuge, and gather the supernatants to the volumetric flask. Dilute the contents of the flask with a mixture of methanol and water (1:1) to volume, and mix.
Chromatographic system
(see
Chromatography  621
621
)—The liquid chromatograph is equipped with a 205-nm detector and a 4.6-mm × 15-cm column that contains packing L1. The flow rate is about 0.8 mL per minute. Chromatograph the
Standard solution, and record the peak areas as directed for
Procedure: the relative standard deviation for replicate injections is not more than 2.0%.
Procedure—
Separately inject equal volumes (about 10 µL) of the
Standard solution and the
Test solution into the chromatograph, record the chromatograms, and measure the areas for the major peaks. Calculate the percentage of
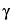
-glutamyl-(
S)-allyl-
L-cysteine in the portion of Garlic taken by the formula:
25(C / W)(rU / rS),
in which
C is the concentration, in mg per mL, of USP
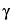
-Glutamyl-(
S)-Allyl-
L-Cysteine RS in the
Standard solution; W is the weight, in g, of garlic cloves taken for the
Test solution; and
rU and
rS are the
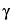
-glutamyl-(
S)-allyl-
L-cysteine peak responses obtained from the
Test solution and the
Standard solution, respectively.