Packaging and storage—
Preserve in tight containers, preferably in a cool place.
Solubility in water—
Not more than 30 mL of water is required to dissolve 1 g at a temperature of 80

.
Identification—
A:
A solution (1 in 50) prepared by heating a uniform dispersion in a hot water bath to 80

(
Solution A) becomes more viscous upon cooling and may form a gel.
B:
To 10 mL of Solution A, while still hot, add 4 drops of potassium chloride solution (1 in 10), mix, and cool. A short-textured (“brittle”) gel indicates a carrageenan of a predominantly kappa type; a compliant (“elastic”) gel indicates a predominantly iota type. If the solution does not gel, the carrageenan is of a predominantly lambda type.
C:
Dilute a portion of
Solution A with about 4 parts of water, and add 2 to 3 drops of
methylene blue TS: a blue, stringy precipitate is formed (also positive for furcellaran, a similar colloid).
D:
Obtain IR absorption spectra on the gelling and non-gelling fractions of the specimen by the following procedure. Disperse 2 g in 200 mL of potassium chloride solution (1 in 40), and stir for 1 hour. Allow to stand for 18 hours, stir again for 1 hour, and transfer to a centrifuge tube. (If the transfer cannot be made because the dispersion is too viscous, dilute with up to 200 mL of the potassium chloride solution.) Centrifuge at approximately 1000 g for 15 minutes.
Remove the clear supernatant, resuspend the residue in 200 mL of potassium chloride solution (1 in 40), and centrifuge again. Coagulate the combined supernatants by adding 2 volumes of dilute alcohol (9 in 10). (Retain the sediment for use subsequently as directed.) Recover the coagulum, and wash with 250 mL of the dilute alcohol. Press the excess liquid from the coagulum, and dry it at 60

for 2 hours: the material so obtained is the nongelling fraction (
lambda carrageenan).
Disperse the sediment retained from the foregoing procedure in 250 mL of cold water, heat at 90

for 10 minutes, and cool to 60

. Coagulate the mixture, then recover, wash, and dry the coagulum as described above: the material so obtained is the gelling fraction (
kappa- and
iota-carrageenan).
Prepare a solution (1 in 500) of each fraction, cast films 5 µm thick (when dry) on a suitable nonsticking surface, and obtain the IR absorption spectrum of each film. Carrageenan has strong, broad absorption bands, typical of all polysaccharides, in the 1000 to 1100 cm
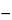 1
1 region. Absorption maxima are 1065 cm
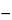 1
1 and 1020 cm
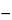 1
1 for gelling and nongelling types, respectively. Other characteristic absorption bands and their intensities relative to the absorbance at 1050 cm
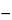 1
1 are as shown in the accompanying table.
Wave Number cm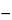 1 1 |
Molecular Assignment |
Absorbance Relative to 1050 cm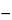 1 1 |
|
|
Kappa |
Iota |
Lambda |
| 1220 to 1260 |
Ester sulfate |
0.7 to 1.2 |
1.2 to 1.6 |
1.4 to 2.0 |
| 928 to 933 |
3,6-anhydrogalactose |
0.3 to 0.6 |
0.2 to 0.4 |
0 to 0.2 |
| 840 to 850 |
Galactose-4-sulfate |
0.3 to 0.5 |
0.2 to 0.4 |
— |
| 825 to 830 |
Galactose-2-sulfate |
— |
— |
0.2 to 0.4 |
| 810 to 820 |
Galactose-6-sulfate |
— |
— |
0.1 to 0.3 |
| 800 to 805 |
3,6-anhydrogalactose-2-sulfate |
0 to 0.2 |
0.2 to 0.4 |
— |
Viscosity  911
911 —
—
Transfer 7.5 g to a tared, tall-form, 600-mL beaker, add 450 mL of water, and disperse with agitation for about 15 minutes. Add water to bring the weight to 500 g, and heat in a water bath, with continuous agitation, until a temperature of 80

is reached. Add water to adjust for loss by evaporation, cool to between 76

and 77

, and place in a constant-temperature bath maintained at 75

. Provide a suitable rotational viscosimeter with a spindle 1.88 cm in diameter and 6.51 cm high, using an immersion depth of 8.10 cm (No. 1 spindle). Allow the spindle to rotate in the solution at 30 rpm for 6 revolutions, then observe the scale reading. Convert the scale reading to centipoises by multiplying by the constant for the spindle and speed employed. The viscosity at 75

is not less than 5 centipoises.
Microbial limits  61
61 —
—
The total bacterial count does not exceed 200 cfu per g, and the tests for
Salmonella species and
Escherichia coli are negative.
Loss on drying  731
731 —
—
Dry it at a pressure not exceeding 10 mm of mercury at 70

for 18 hours, cool in a desiccator, and weigh: it loses not more than 12.5% of its weight.
Acid-insoluble matter—
Transfer about 2 g, accurately weighed, to a 250-mL beaker containing 150 mL of water and 1.5 mL of sulfuric acid. Cover with a watch glass, and heat on a steam bath for 6 hours, rubbing down the wall of the beaker frequently with a rubber-tipped stirring rod, and replacing any water lost by evaporation. Transfer about 500 mg of a suitable filter aid, accurately weighed, to the beaker, and filter through a tared filtering crucible provided with a 2.4-cm glass fiber filter. Wash the residue several times with hot water, dry at 105

for 3 hours, cool in a desiccator, and weigh. The difference between the total weight and the sum of the weights of the filter aid, crucible, and glass fiber filter is the weight of the acid-insoluble matter. It is not more than 2.0% of the weight of Carrageenan taken.