Physicians frequently prescribe special formulations of noncommercially available drugs for patient care. Upon receipt of a prescription for such a preparation, pharmacists (or other qualified individuals working under the authority and supervision of a physician) compound the drug formulation and dispense it to the patient. For convenience, a limited bulk quantity of the special formulation may be compounded in anticipation of future dispensing requirements. Such medical and pharmacy practices are regulated by state boards of medicine and pharmacy. Physicians who prescribe a drug that must be compounded extemporaneously bear the professional responsibility to base its use on sound scientific and medical evidence. Pharmacists and physicians who compound (or oversee the compounding of) drug preparations on prescription orders, bear the professional responsibility to ensure that the preparation meets prescribed and appropriate standards of strength, quality, and purity.
Radiopharmaceuticals administered for positron emission tomography (PET) procedures typically incorporate radionuclides that possess very short physical half-lives, T½ (e.g., T½ of 18F = 109.7 minutes, of 11C = 20.4 minutes, of 13N = 9.96 minutes, and of 15O = 2.03 minutes). As a result, these radionuclides are usually produced using particle acceleration techniques (e.g., cyclotron) at or within close proximity to the site where the PET procedure will be conducted. The radionuclides may then be synthetically incorporated into the final PET radiopharmaceutical for subsequent patient administration.
Control of Components, Materials, and Supplies
The following activities are to be established and performed. A designated person shall be responsible for ensuring that these activities are carried out and completed properly.
(1) Establish written specifications for
-
the identity, purity, and quality of components (including ingredients, reagents, target solutions, and gases); the identity and quality of containers and closures, and other materials (e.g., transfer lines, purification devices, membrane filters) that come into contact with the final PET radiopharmaceutical; and the identity, purity, and quality of analytical supplies (e.g., solvents, chromatography columns, and reference materials), sterility test media, endotoxin test reagents, and other supplies intended for use in PET radiopharmaceutical quality control procedures; and
-
the appropriate storage (i.e., based on heat, light, and humidity considerations) of components, containers and closures, materials and supplies used for the compounding of PET radiopharmaceuticals.
(2) Log-in each lot of shipments of components, containers and closures, materials, and supplies used for the compounding of PET radiopharmaceuticals, and record the date of receipt, quantity received, manufacturer, lot number, and expiration date. If no expiration date is designated by the manufacturer, an expiration date is to be assigned to the component, material, or supply based on knowledge of its physical and chemical properties and prior experience with its use. For organic substrates, reactants, and reagent materials that are potentially susceptible to degradation or to a change in composition, the expiration date is based on the component's documented evidence of stability.
(3) Determine that each batch of components, containers and closures, materials, and supplies used for the compounding of PET radiopharmaceuticals are in compliance with established written specifications. A reliable manufacturer is routinely used as the source of a given product. Certification of compliance with the specifications for containers, closures, and materials marketed commercially for the intended purpose(s) may be accomplished by inspection of the product labeling and/or inspection of the certificate of analysis provided by the manufacturer. Certification of compliance with the specifications for other components and materials used in the compounding of PET radiopharmaceuticals may be accomplished by inspection of the certificate of analysis provided by the manufacturer. The identities of each lot of components, containers and closures, and materials used in the compounding of PET radiopharmaceuticals are to be verified by defined procedures, tests, and/or documented certificates of analysis, as appropriate.
(4) Store components, containers and closures, materials, and supplies used for the compounding of PET radiopharmaceuticals in a controlled access area according to established storage conditions.
Compounding Procedure Verification
The following activities are to be established or performed. A designated, qualified, and trained person shall be responsible for ensuring that these activities are carried out and properly completed by qualified and trained personnel.
(1) Written acceptance criteria for the identity, purity, and quality of each PET radiopharmaceutical being compounded. If a USP monograph exists for a particular PET, then these standards are the minimum acceptance criteria (see Official and Official Articles under the General Notices and Requirements).
(2) Written and verified procedures for the compounding of each PET radiopharmaceutical that
-
incorporate, for each PET radiopharmaceutical intended for parenteral administration, sterile membrane filtration (0.22 µm);
-
incorporate, for each PET radiopharmaceutical intended for inhalation, particulate filtration (0.45 µm); and
-
are routinely updated and verified as changes in the compounding procedures are implemented or are reviewed and verified at a minimum of once a year to ensure that they are current. A master file of written compounding procedures currently used for each PET radiopharmaceutical is to be maintained within the PET facility. Copies of outdated compounding procedures shall also be retained, separate from the master file, for review purposes.
(3) Appropriate controls over computer and related automated equipment to ensure that changes in compounding software are instituted only by authorized personnel, that such changes are documented and verified, and that only current versions of the software are available and used in PET radiopharmaceutical compounding procedures. A diskette copy and printout of current computer software programs used in the compounding of each PET radiopharmaceutical is to be maintained within a master file located in the PET facility. Copies of outdated computer software programs shall also be retained, separate from the master file, for review purposes.
(4) Verification studies to ensure that the written compounding procedures, computer software program, equipment, and facilities result in a PET radiopharmaceutical that meets established acceptance criteria. Such verification studies must
-
include documented evaluations of the radiochemical identity and purity, radionuclidic identity and purity, specific activity, sterility (for parenteral agents), bacterial endotoxins (for parenteral agents), pH, osmolality (for parenteral agents), if appropriate, appearance, stereochemical purity (for applicable compounds), potential organic volatile impurities, other toxic chemicals that may have been used during the synthesis or purification procedure, effective concentration of a stabilizer (if any), chemical purity of the PET radiopharmaceutical [NOTE—Evaluations for chemical purity must include analyses for the presence of starting materials, known intermediates, by-products, and known degradation products], and equivalency of initial and final sub-batches (for PET radiopharmaceuticals with radionuclides having a T½ < 20.0 minutes). For purposes of this chapter, “sub-batch” is defined as a quantity of PET drug product having uniform character and quality, within specified limits, that is produced during one succession of multiple irradiations, using a given synthesis and/or purification operation; and
-
be signed, and dated, and retained as an indication that the compounding procedures, equipment, and facilities have resulted in a PET radiopharmaceutical that meets established acceptance criteria.
Whenever there is a change in the compounding procedures, computer software program, or component specifications that has the potential to alter the identity, quality, or purity of the drug product, verification procedures and studies must be conducted. Verification studies on a minimum of three consecutive batches, which show that the product meets acceptance critera, are to be performed prior to the approval, for human use, of new or revised compounding procedures for a given PET radiopharmaceutical. For routine verified processes that are being used with consistent success, a minimum of one verification study that shows the product meets acceptance criteria must be conducted on an annual basis.
PET Radiopharmaceutical Compounding for Human Use
The following are to be performed according to established written procedures and documented. A designated, qualified, and trained person shall be responsible for ensuring that these activities are carried out and completed properly by qualified and trained personnel.
(1) Inspect the compounding and dispensing area and all equipment for cleanliness and suitability immediately before use. Before initiating compounding and dispensing activities, extraneous materials and labels must be removed from involved areas and equipment. For PET radiopharmaceuticals intended for parenteral administration, all manipulations of components, containers and closures, and materials distal to sterile membrane filtration must be performed using an appropriate aseptic technique in an appropriately controlled environment.
(2) Ensure the correct identity, quantity, and suitability of components, containers and closures, and other materials used in compounding the PET radiopharmaceutical.
(3) Label all subdivided components used in the compounding procedure for identity and traceability.
(4) Label the final PET radiopharmaceutical container or dispensing-administration assembly prior to initiating the compounding procedure. The following information must appear on the label or labeling attached to the final container or dispensing-administration assembly: the identity of the PET radiopharmaceutical, and added substances (e.g., stabilizers and preservatives), an assigned batch or lot number, and the required warning (e.g., radioactive) statements or symbols. The final PET radiopharmaceutical shall also be labeled to include the total radioactivity and radioactive concentration at the stated time of calibration, the expiration time and date, and any required or applicable warning statements (e.g., “Caution-Radioactive Material”, “Do not use if cloudy or contains particulate matter”) and/or the radioactivity symbol.
(5) Compound the PET radiopharmaceutical according to current, verified procedures. A written record must be maintained for each batch (i.e., the material produced during a single synthesis and purification) of the compounded PET radiopharmaceutical. This written record includes
-
lot numbers, manufacturer identities, expiration dates, and quantities of all components, containers and closures, and materials used in the compounding procedure;
-
a description of the individual compounding procedures to be followed;
-
the initials of the responsible individual indicating that the compounding procedure for the batch is an accurate reproduction of the current, verified compounding procedure;
-
the initials of the responsible individual indicating that critical steps and processes in the compounding procedure were completed [NOTE—Critical steps in automated compounding processes shall be monitored through direct observation (if possible, considering visual or radiation exposure constraints) or via computer or other feedback mechanisms];
-
documentation of the investigation of any unplanned deviations in, or unexpected results of, verified compounding procedures or processes, including documentation of the outcome of the investigation;
-
the percent yield calculated on the basis of the known or expected decay-corrected amount of the starting radionuclide that is synthetically incorporated into the final radiopharmaceutical;
-
raw analytical data on each batch of compounded PET radiopharmaceutical; and
-
the date and the signature of the individual assuming overall responsibility for, and adherence to, the verified compounding procedure.
Quality Control
The following are to be performed according to established, written procedures and documented. A designated, qualified, and trained person shall be responsible for ensuring that these activities are carried out and completed properly by qualified and trained personnel.
(1) Establish, in writing, the quality control tests to be performed on individual batches of the PET radiopharmaceutical, the analytical procedures, and the corresponding acceptance criteria.
-
For PET radiopharmaceuticals labeled with a nuclide having a
T½ 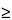
20.0 minutes, the following quality control procedures are to be performed on each batch (i.e., the material produced during a single synthesis and purification operation) prior to release: measurement of the pH of parenteral and oral dosage forms; visual inspection of parenteral and oral dosage forms; determination of the radiochemical purity and identity of all dosage forms; determination of the radionuclidic identity of all dosage forms; and assessment of the specific activity of PET radiopharmaceuticals with mass-dependent localization or toxicity concerns; and evidence of compliance with the established acceptance criteria for residual solvents and other toxic chemicals used during the synthesis or purification procedures.
-
For PET radiopharmaceuticals labeled with a nuclide having a T½ < 20.0 minutes, a batch is defined as all related sub-batches of the PET radiopharmaceutical compounded during a given day. The following quality control procedures are to be performed on an initial quality control sub-batch of each such PET radiopharmaceutical prior to release for human use of subsequent sub-batches: measurement of the pH of parenteral and oral dosage forms; visual inspection of parenteral and oral dosage forms; determination of the radiochemical purity and identity of all dosage forms; determination of radionuclidic identity of all dosage forms; and assessment of the specific activity of PET radiopharmaceuticals with mass-dependent localization or toxicity concerns; and evidence of compliance with the established acceptance criteria for residual solvents and other toxic chemicals used or produced during the synthesis or purification procedures.
-
For each batch of PET radiopharmaceutical intended for parenteral administration, perform a membrane filter integrity test immediately after completion of product filtration. This post- filtration integrity test is to be completed prior to release of the batch for human use, except in the case of 15O water, where it may be necessary to release the batch prior to completion of the post-filtration integrity test. In this case, the test is completed as soon as possible after release of the batch.
-
For PET radiopharmaceuticals intended for parenteral administration, perform an in-process 20-minute endotoxin “limit test” (i.e., incorporating positive controls in the range of 5 EU per mL to 175 EU/
V, where
V is the maximum volume of injection) on each batch (
T½ 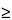
20.0 minutes) or quality control sub-batch (
T½ < 20.0 minutes) of the radiopharmaceutical prior to release, for human use, of the batch or subsequent sub-batches.
-
For PET radiopharmaceuticals intended for parenteral administration, a standard 60-minute bacterial endotoxin test must be performed on each batch (
T½ 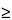
20.0 minutes) or quality control sub-batch (
T½ < 20.0 minutes) of the radiopharmaceutical. Endotoxin testing may also be performed using other recognized procedures (see
Bacterial Endotoxin Test  85
85
). Regardless of which test is utilized, an assessment of the bacterial endotoxins should be performed prior to release of each batch (
T½ 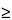
20.0 minutes) or quality control sub-batch (
T½ < 20.0 minutes) of the radiopharmaceutical before release for human use of the batch or subsequent sub-batches.
-
Sterility tests for each PET radiopharmaceutical intended for parenteral administration are performed on each batch (
T½ 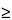
20.0 minutes) or quality control sub-batch (
T½ < 20.0 minutes). Sterility tests are also performed following the replacement of system components. Sterility tests are initiated within 24 hours of sterile filtration. Product samples are tested individually and are not pooled.
(2) Establish written procedures for the performance of quality control tests on batches of PET radiopharmaceuticals intended for human use.
(3) Conduct verification testing of equipment and procedures used for the quality control testing of PET radiopharmaceuticals. Using internal or external standards, the correct operation of analytical equipment, such as gas chromatography or high-performance liquid chromatography (see
System Suitability under
Chromatography  621
621
) must be confirmed upon initial installation or upon major repair. Correct operation of analytical equipment must also be checked (i.e., a system suitability test must be performed) on a scheduled basis, and maintenance must be performed according to appropriate, written, scheduled procedures. Dose calibrators used in measuring the bulk radioactivity and the radioactivity of dispensed dosages of PET radiopharmaceuticals should be tested in accordance with applicable state regulations governing the medical use of radioactive materials.
(4) Perform quality control tests on batches of PET radiopharmaceuticals according to written procedures, and initial the results of such testing.
(5) Accept or reject the individual batch of the PET radiopharmaceutical based on the conformity of quality control test results with established acceptance criteria. If the individual batch of the PET radiopharmaceutical is acceptable, sign and date the batch.
(6) Investigate unacceptable quality control test results and document the outcome of such investigations.
Sterilization and Sterility Assurance
A complete system of process controls is required to assure sterility of PET radiopharmaceuticals. Sterilization activities for the following elements of the process are to be established, documented, and performed.
Compounding Equipment and Components—
Equipment used to prepare PET radiopharmaceuticals must be properly cleaned and kept in sanitary condition. Equipment in contact with a PET drug solution may be processed to remove endotoxin and may be sterilized to eliminate bioburden. Prepared equipment is stored and protected to maintain cleanliness and, if necessary, sterility. It is recommended that components for PET products be obtained from qualified suppliers after verifying that the components meet specifications for sterile drug products. It is further recommended that sterile vials, syringes, transfer sets, and filters be obtained from commercial sources. If components are sterilized by the PET facility, the sterilization processes and asepsis of assembly components must be verified. Verification of sterilizer performance must be repeated periodically. Solutions for parenteral administration must be filter-sterilized and aseptically transferred to a sterile, nonpyrogenic, multiple-dose vial. Certain finished dosage forms of PET products may not be transferred to a vial and require special consideration.
Environmental Controls—
The work area used for compounding the finished dosage form must be clean. The aseptic hood is protected from sources of microbial contamination and is located in an area where surrounding personnel traffic is controlled and limited. Appropriate clean laboratory clothing shall be worn when performing functions in the aseptic hood. Components, materials, and equipment are transferred to the aseptic area in protective wrapping or containers. Aseptic techniques are used whenever a sterile solution dosage form is handled. The containers, filter assembly, vent filters, and needles for the final dosage form must be sterile, disposable, and for single use only. After the filter and the product container are assembled, the sterility of this assembly must be preserved. If the sterility of any component is compromised, the component or set must be replaced. Before penetrating the finished product container, the septum of the product vial must be thoroughly swabbed with a disinfectant solution (i.e., freshly filtered or certified sterile 70% alcohol) and allowed to air-dry in the aseptic hood.
Aseptic Hood—
Assemble the product filter and the container and closure system for the finished product in an aseptic hood. Sterile (aseptic) operations should be conducted within an aseptic workstation with an air cleanliness rating of class 100 (e.g., Laminar flow hood or isolator). The aseptic hood surfaces and equipment surfaces allow easy cleaning and disinfecting. Disinfectants are filtered or certified sterile with a manufacturer's certificate of analysis, and the hood's internal surfaces are cleaned and disinfected daily before use and after new equipment is brought in. Microbiological testing of the aseptic hood is performed periodically (e.g., weekly). This may be done by swab or contact plate for surfaces and settle plate or dynamic air sampler. Airborne, nonviable particle counting may be performed less frequently.
Aseptic Technique—
All aseptic operations, including the assembly of sterile components, compounding, filtration, and manipulations of sterile solutions must be performed by operators qualified to work with aseptic techniques. Aseptic manipulations shall be performed using sterile items sealed in protective covering and opened within the aseptic hood. Any sterile equipment or component that is compromised by contact with a nonsterile surface must be replaced. Sterile components shall be transferred from the hood with closures in place. Aseptic operations are performed by operators wearing laboratory clothing appropriate for pharmaceutical compounding. Gloved hands are disinfected immediately before reaching into the aseptic hood.
Aseptic area operators are trained and evaluated periodically through observation as well as through microbiological tests. Aseptic techniques used to make sterile products are evaluated by simulations, in which a microbiological growth medium is substituted for the PET radiopharmaceutical solution. Process simulations include manipulations such as connecting vents and filtration. Verification of the medium's growth promotion capability in the PET drug container is an essential control for process simulations. After completing the simulation process, the final product container is gently shaken to permit the medium to contact all surfaces, and the container and the medium are incubated (at 30

to 35

, 20

to 25

, or another suitable temperature) for 14 days with periodic examination for evidence of growth: the absence of growth in the containers is necessary for an acceptable test result. Simulations are performed in triplicate to qualify a new operator. Each operator repeats one simulation about once a year or any time procedures are changed.
Qualification of the Filtration Process—
Sterilizing filtration is the final safeguard in removing microorganisms from solutions of PET radiopharmaceuticals. This critical procedure requires that microbial retention by membrane filters be demonstrated under specified conditions. Filters must not release particles or soluble compounds, bind product ingredients, or lose integrity during use. When the filters are prepared and sterilized by a commercial filter manufacturer, the filter manufacturer generally provides filtration conditions (i.e., pressure and flow rate); these conditions are not to be exceeded when preparing a PET drug product. For most aqueous solutions of near neutral pH, certification regarding microbial retention challenges to the selected filter may be obtained from the filter manufacturer. Certification of conformance to specifications must be examined and maintained for each filter lot.
Before using filters from a particular lot, a sample is tested for integrity to demonstrate that the membrane and housing have not lost the ability to retain microorganisms. The manufacturer's recommended method or an alternative method, if demonstrated to be acceptable, may be used.
The sterilizing membrane filter must also be tested for integrity after filtering the compounded PET radiopharmaceutical but before the product is released. An example of a simple test is the “Bubble Point” test, which uses a pressure gauge and a source of air pressure connected to the transfer set attached to the filter. The filter disk is placed in a beaker of water with the filter outlet below the water surface, and air pressure is applied gently to the nonsterile side of the filter assembly. The air pressure is increased until the validated bubble point is reached, at which point the pressure is maintained briefly to allow equilibration. Filter integrity is demonstrated to be acceptable in the absence of a steady stream of bubbles.
Microbiological Testing of Finished Products—
PET radiopharmaceuticals for parenteral administration must be sterile and free of endotoxins, as demonstrated by sterility and endotoxin tests. Endotoxin tests are initiated promptly after compounding, and sterility tests are started no later than 24 hours after compounding. Each lot shall be assayed individually and not pooled with other lots. If a microbiological test fails, an investigation shall be undertaken to identify the cause, and corrections shall be undertaken. After a record of successful sterility tests is established for a particular PET drug, only the first lot prepared each day shall be subject to a sterility test using cultivation methods. However, when a different PET drug is made at the facility or a new lot of sterile components (for example, filter or final product container) is substituted, then the first daily lot of that PET drug is tested for sterility.